Tumour Heterogeneity and Immune Escape: Circumventing Some of the Biggest Roadblocks in Cancer Treatment

Some of the biggest complications in cancer therapy arise from the tumour not responding to appropriate treatment. Two major obstacles in this context are tumour heterogeneity and immune escape, which both featured in presentations at Oxford Global’s Biomarkers 2023 event. Here, we explore how these issues can frustrate treatments for cancer, and how in turn researchers are working to overcome these hurdles to save the lives of cancer patients.
How Does Tumour Heterogeneity Affect Immunotherapy?
Tumour heterogeneity refers to genetic, phenotypic, and behavioural differences which may be displayed by cancer cells within a single tumour. These variations mean that not all cancer cells in a tumour may respond in the same way to a given treatment. Tumour heterogeneity can complicate immunotherapeutic approaches, as a therapy that targets a specific type of cancer cell may not be effective against other cell types in the same tumour.
Cancer cells can also evolve and adapt over time. This can lead to a phenomenon known as immune escape, where treatment resistant clones that can evade the host’s immune response. Heterogeneity in a tumour can affect the accuracy of diagnostic tests such as biopsies, which may not sample every cell type within a tumour. This may result in an incomplete understanding of the cancer’s genetic and phenotypic profile, which can influence treatment decisions.
The treatment complications posed by tumour heterogeneity are a main focus for the Institute of Cancer Research in London. In particular, intra-tumoural heterogeneity between patients is a tricky hurdle. In this context, ‘intra’represents heterogeneity between the individual patient tumour heterogeneities. Although they may all be linked to each other, some will respond differently.
One example would be two patients who receive the same treatment for the same cancer. While one patient sees their tumour shrink, the other may experience toxicity and no change in the status of their tumour. This means that there are different levels of stratification one could do to visualise the tumour.
Accounting for Intra-Tumoural Complexity
This complexity in interpreting tumoural condition extends to attempts to interpret tumoural condition, as different imaging resolutions will highlight different variations. Researchers can study different kinds of immune cells and cancer cells at the spatial level through multiplex immunohistochemistry. However, integrating this heterogeneity across tumours requires the reconciliation of a plethora of factors.
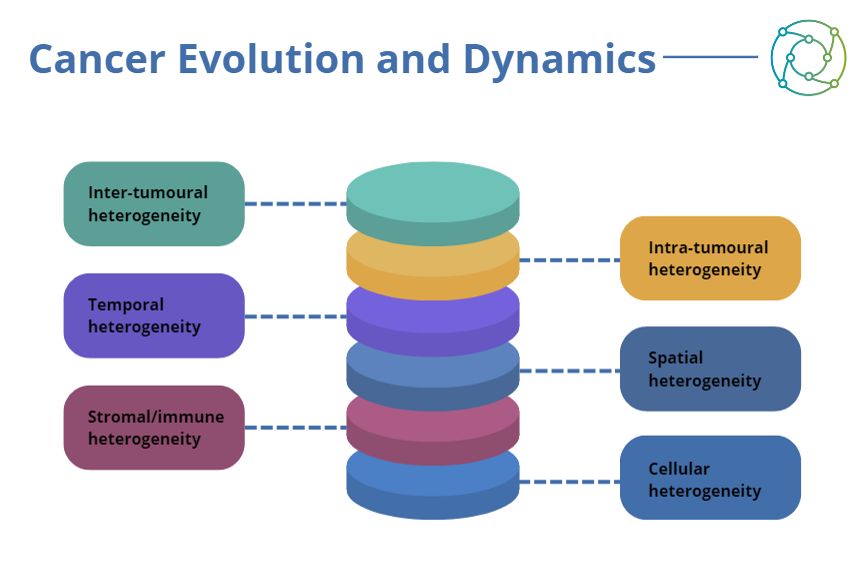
Researchers looking to investigate tumour heterogeneity need to account for spatial, temporal, stromal, and cellular heterogeneity aspects in addition to intra-tumoural heterogeneity. Many elements need to be integrated at the systemic level for researchers to understand how such tumours actually progress. Consideration also needs to be made for the highly dynamic nature of cancers, necessitating allowance for temporal variations in tumour property and behaviour.
In the face of such variability, the best approach is personalised medicine: if no two patients may respond in the same way to the same treatment, then taking individual tumour heterogeneity into account is a better way of pursuing treatment success. Personalised medicine involves matching patients to a specific therapy based on an approach more likely to succeed, with an evaluation made through multi omics techniques. Biomarkers offer a good opportunity for matching patients with appropriate therapies.
One example of this approach in action involves treatment approaches to triple-negative breast cancer (TNBC), with the stratification of different subtypes based on gene expression in an immune context. TNBC subtypes show differential prognosis and association with tumour-infiltrating lymphocytes (TILs). By validating the multi-omics pathway, researchers have found a subpopulation which exhibited signalling associated with higher risk.
Confronting Immune Escape in Cancer Therapy
Equally tricky to address is the issue of immune escape — a phenomenon where cancer cells evade the body’s immune system and continue to proliferate. Typically, the immune system identifies and eliminates abnormal cells such as cancer cells. However, cancer cells can develop mechanisms to avoid detection or destruction by the body’s immune system. One way this is done is through the downregulation of antigen expression on their surface.
- Improving patient selection approaches through immuno-oncology biomarkers
- How can cancer screening approaches benefit from AI?
- Qualifying clinical trial validation for biomarkers in NASH and liver cirrhosis
Immune escape can complicate attempts at cancer treatment, as immunotherapies may not prove effective for patients whose cancer cells have developed mechanisms of immune escape. Immunotherapies work by boosting the body’s immune response against cancer cells, so immune escape actively confounds this approach. To address immune escape, researchers and treatment providers are developing new strategies to enhance the immune system’s ability to recognise and attack cancer cells.
Accounting for Population Variability in Immunotherapeutic Approaches
Across the immunotherapeutic field, an understanding of the incidence of cancer subtypes and genetic variability over populations is also a key consideration. There exists a marked disparity in the incidence of different subtypes of breast cancer between India and the USA as one example of this. While rates of HR+HER2- are identical across patient populations, incidences of TNBC are twice as prevalent in India compared to the USA. This could be attributed to biology, or it could be the way patients are screened by the healthcare system which leads to delayed diagnosis.
An investigation by the Institute of Cancer Research in London into gene expression subtypes associated with TNBC found that the biology was not different between Indian and Western populations, suggesting there may potentially be other factors which predicate patient susceptibility to disease on a geographic basis. While the complexities associated with identifying tumour state and coordinating an effective immunotherapeutic response are many, pioneering healthcare providers are rising to the challenge.
Want to learn more about the applications of biomarkers in neurological pathology? Head over to our Biomarkers portal for the latest news on advances in the field and insights from the industry’s best and brightest. If you’d like to learn more about our upcoming Biomarkers US conference, visit our event website to download an agenda and register your interest.