The Applicability of Clinical Biomarkers for NASH and Fibrosis

Presented by Archana Vijayakumar, Research Scientist of Fibrosis at Gilead
Edited by Tia Byer
Non-alcoholic fatty liver disease is a progressive disease which begins as a benign condition characterised by increased lipid accumulation and hepatocytes. This then develops into something more inflammatory such as non-alcoholic steatohepatitis or NASH. “At this stage, you see progressive inflammation, hepatocyte ballooning, degeneration, and mild fibrosis,” Archana Vijayakumar, Senior Research Scientist I of Fibrosis at Gilead, explained at Oxford Global’s Biomarkers UK 2021.
The NASH further progresses towards cirrhosis, where there is an accumulation of fibrotic scar tissue characteristic of F3 and F4 fibrosis. “The pattern of the scaring usually takes the form of a chicken wire pattern,” Vijayakumar continued (see figure 1). Cirrhosis often results in the patient needing a liver transplant.
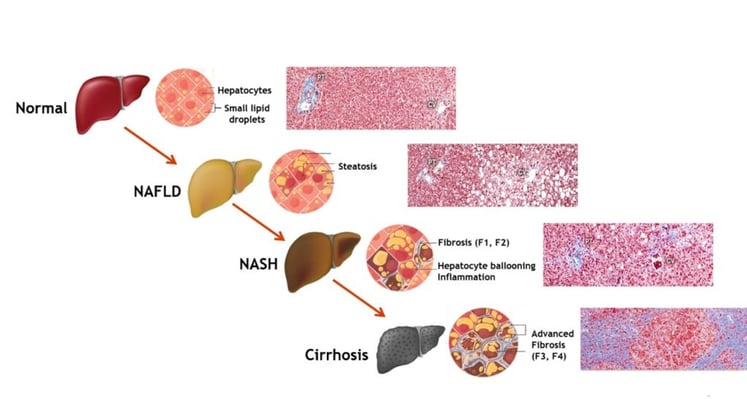
Figure. 1 – Understanding NAFL and NASH disease development
Fibrosis and Clinical Outcomes in NASH
Fibrosis is associated with clinical outcomes in NASH. Referencing a study conducted by Angulo P et. al (2015), Vijayakumar explained how “survival curves for subjects with varying stages of fibrosis showed clear associations with clinical outcomes, whilst starting from an F0 or essentially no fibrosis, the survival rates for liver transplants drops drastically” (figure 2). The referenced study found little association with steatosis (a largely harmless build-up of fat in the liver cells), whilst portal inflammation and hepatocyte ballooning were associated with notably worse survival rates.
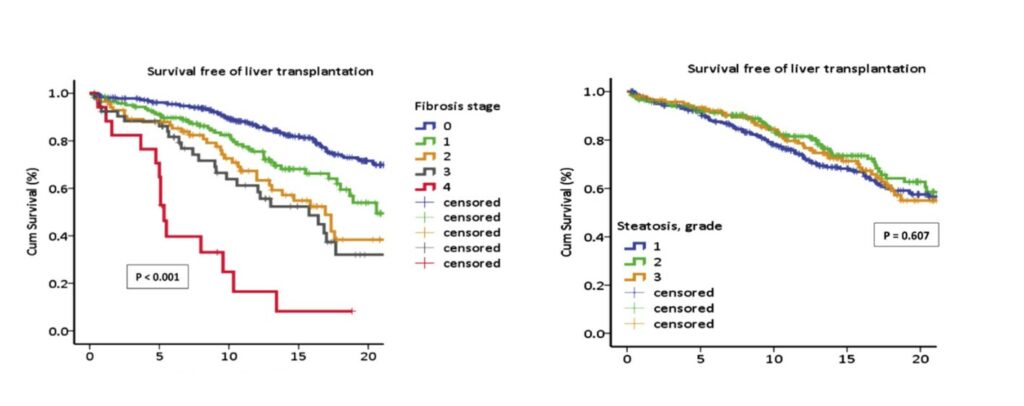
Figure. 2 - Angulo P et. al Gastroenterology. 2015 Aug;149(2):389-97.
“Liver biopsy is the current gold standard for definitive NASH diagnosis and clinically approval endpoints to assess therapeutic efficacy,” Vijayakumar pointed out. However, this approach is wrought with disadvantages; liver biopsy is a “risky procedure, especially in advanced patients where the risk of bleeding is significantly higher.” It is also costly, requiring expertly trained technicians to be able to sufficiently biopsy subject.
An additional limitation is the possibility of sampling variability, where two different liver regions may not have the same level and degree of fibrosis. “Besides, different pathologists reading the liver sections could also lead to variable interpretations and different indices scores,” Vijayakumar continued. As a result, the field is working hard to develop non-invasive biomarkers to test and evaluate the spectrum of disease progression in NASH and fibrosis.
Non-Invasive Tests and Biomarkers as Diagnostic and Prognostic Tools
Several non-invasive image-based tests and plasma biomarkers are now under investigation. These imaging-based tools include ultrasound and MRI scanning, the latter of which is used for a definitive steatosis diagnosis. “There is even data showing that greater than or equal to a 30% reduction in liver fat by the quantitative marker MRI -PDFF is actually associated with improved efficacy,” Vijayakumar explained.
“Liver biopsy is the current gold standard for definitive NASH diagnosis and clinically approval endpoints to assess therapeutic efficacy”
Serum-based biomarkers include the fatty liver test, the Steato Test, and the NAFLD liver test, all of which predict the presence and not the severity of steatosis. Progressing towards NASH are several biomarkers currently being evaluated for their diagnostic abilities. These include the cytokeratin 18 fragments, M30 and M65, and the Enhanced Liver Fibrosis (ELF test) which perform modest accuracy and function as markers for hepatocyte degeneration.
One of the most popular markers for fibrosis is transient elastography, a non-invasive imaging-based tool used to assess liver stiffness. “Studies have shown that greater than or equal to 25% reduction in liver stiffness is associated with improved clinical outcomes,” Vijayakumar said. There are also several exploratory biomarkers that are currently under evaluation. These novel alternative technologies include the SOMAscan platform, SERS technology, and the Glyco Liner Profile technology.
Gilead’s ATLAS Trial
Gilead has an active NASH programme and focuses mainly on advanced fibrosis akin to F3 and F4 stages. During their ATLAS trial run over a period of 48 weeks, Gilead evaluated the efficacy of monotherapy and combinations of firsocostat and cilofexor. Firsocostat is an acetyl CoA carboxylase (ACC) inhibitor. Cilofexor, is a farnesoid X receptor (FXR) agonist, in NASH subjects.
“Based on the histological endpoints, we found that the firsocostat combination resulted in a greater number of patients having one-point stage improvements in fibrosis” Vijayakumar explained. “Although the results did not reach statistical significance, the data did form the basis of a longer phase II study in collaboration with Novo Nordisk, which is currently enrolling subjects.” The cilofexor combination had a greater response rate when looking at NASH resolution without fibrosis worsening.
- Treating the Untreatable: Unlocking Precision Medicine Biomarkers for NASH
- Overcoming Challenges of NASH in Clinical Development
- Non-Invasive Biomarkers: Aiding Clinical Qualification of Prognostics and Monitoring Biomarkers for Liver Cirrhosis
“Gilead’s goal within the preclinical stage was to evaluate changes in biomarkers using choline-deficient rat models, with a high-fat diet, to mimic human fibrotic development,” Vijayakumar said. The biomarkers tested included the serum-based alpha-2 macroglobulin and CK-18 fragments. Similar to their histological results, Gilead found combination biomarkers to be the most efficacious.
The ACC and FXR combination was most efficacious at reducing histologic and biomarker enzymes in rats. However, the effects of the monotherapies were amplified in rodents compared to the ATLAS study, Vijayakumar confirmed. “The efficacy of ACC combined with FXR in an extended eight-to-18-week intervention in choline-deficient rodents correlated better with clinical data. At Gilead, we have implemented the learning derived from these studies into evaluating future combinations with some of our newer therapeutic agents,” she summarised.
Conclusions
There is a great need to identify biomarkers of NASH and fibrosis to minimize the variability observed with classical histology-based readouts. Current biomarkers for NASH have demonstrated great utility for identify patients with advanced disease; however, their predictive value in revealing treatment responses are still unclear. Further, understanding the dynamics of these biomarkers in various preclinical models may help identify appropriate models to study different stages of disease and/or predict therapeutic effect of various treatments.
Want to stay up to date with the latest Biomarker news? Register now for Oxford Global’s flagship event, Biomarkers For NASH Symposium. This is a must-attend forum covering the latest trends transforming biomarker and translational research.