Patient Engagement in Biomarker Development

In November 2022, Oxford Global’s Biomarkers series was delighted to host a panel of industry experts for the Discussion Group, Patient Engagement in Biomarker Development. The group came together for one hour of conversation on strategies to improve the clinical adoption and utility of biomarkers in patient care. Notable attendees included senior representatives from Alexion Pharmaceuticals, Boehringer Ingelheim, Janssen, and Novartis.

Meet the Panel
Christopher Conn, Director of Diagnostics Strategy at Amgen*,moderated the session. As Director, he is responsible for developing and implementing end-to-end diagnostic development programs spanning early to late-phase clinical development. Conn has over 15 years of cross-functional experience in the diagnostic and pharmaceutical industries, from product concept development to commercialisation. Conn has a PhD in cell and molecular biology from the University of Cincinnati College of Medicine and has completed postdoctoral training at St. Jude Children's Hospital and the University of Colorado/HHMI.

Joining Conn was George Kumar, Director and World Wide Medical Lead for Biomarkers, Diagnostics and Digital Pathology at Bristol Myers Squibb*. Dr. Kumar currently leads the global PD-L1 training program and various artificial and augmented intelligence (AI) initiatives at BMS. In 2021, he completed his post-graduate certificate program in High-Impact Cancer Research from Harvard Medical School in Boston, MA, USA. He obtained his Ph.D. from the Max Planck Institute for Biological Intelligence in Seewiesen, Germany, followed by post-doctoral work at the European Molecular Biology Laboratory, Heidelberg, Germany, and the University of Wisconsin Medical School/ and Dept. of Electrical and Computer Engineering, Madison, WI USA. His recent book “Predictive Biomarkers in Oncology- Applications in Precision Medicine (eds. Badve S and Kumar GL)” was published by Springer Nature in 2019-20. Dr. Kumar is passionate about using digital pathology and AI for PD-L1 education, training, quality control & assurance, and telepathology in developing countries.
Industry Overview and Digital Biomarkers
In 1998, the National Institutes of Health Biomarkers Definitions Working Group defined a biomarker as “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic response to a therapeutic intervention.”
- The Patient-focused ‘Paradigm Shift’ That Could ‘Revolutionise’ Clinical Trials
- Digital Biomarkers: Using Smartphones for the Passive Monitoring of Neurodegenerative Diseases
- Understanding the Clinical Utility of Digital Biomarkers
In other words, “biomarkers tell you how the body’s doing, and they are measurable,” Kumar confirmed. An example biomarker would be blood pressure which provides a key health indication for how the heart and circulatory systems function. Whilst genomics and protein immunohistochemistry have led the way in developing biomarkers, other molecular measurements, such as metabolic and microbiomics, are still in the preliminary stages of development. Kumar identified digital biomarkers as the area that involves the most patient engagement and interaction. Digital biomarkers refer to continuous remote data streams taken from wearable sensors and mobile devices that guide treatment decisions. By tracking activity per day and risk susceptibility to heart attacks or strokes, wearable digital devices offer a common ground whereby both patient and clinician can access and interpret data.
Patient Treatment Pathway: Clinical Development and Pre-Biomarker Approval
Following the brief overview of the biomarker industry, Conn introduced the topic of patient engagement during clinical development. He began by drawing the audience’s attention to a diagram outlining the biomarker discovery pathway for the approval of companion diagnostics (see figure 1).
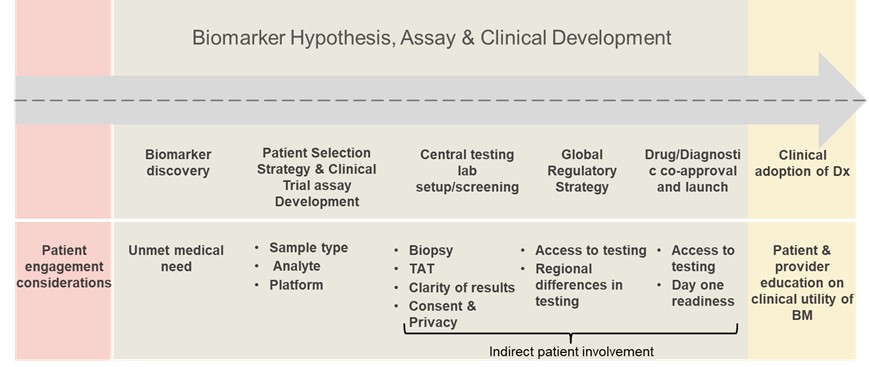
“Of course, there is limited patient engagement at this point,” Conn explained. “When we think about the current unmet medical needs, one thing that comes to mind is this push to develop drugs in the paediatric setting.” In particular, Conn referred to the FDA’s recent investment in asking for the utility of targeted therapy to be explored in the paediatric setting in a biomarker-driven fashion. This means that regardless of histology, there is a need to consider the patient’s perspective within a larger biomarker context.
Conn also mentioned the pressing concern of using non-invasive sampling techniques during the clinical development stage. “From a patient's perspective, a non-invasive procedure such as ctDNA is preferable, but this can greatly impact the quality of the treatment down the line,” he said. Therefore, indirect patient involvement via sampling technique figures as a crucial determiner in later-stage clinical success and adoption.
Patient Treatment Pathway: In Clinic After Biomarker Approval
Post-approval of a companion diagnostic, patient engagement considerations are restricted to the sample (see figure 2). As Kumar put it, “during this stage, all protocols are performed by the pathologists. Approximately 99% of pathologists do not see a patient.”
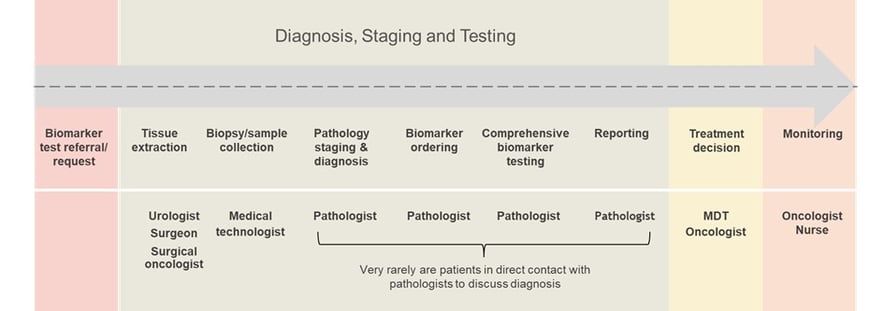
Whilst some more curious patients may actively seek to discuss their diagnosis with the pathologist and ask for a report, this is not common. “The patients rarely get involved with biomarker ordering or testing,” Kumar continued. Drawing on an example of a cancer biopsy, Kumar explained how once routine reporting is finished, the process is handed over to the oncologist and tumour board. Only then will a final decision be made over which therapeutic drug to administer and method of monitoring to adopt.
Improving Patient Involvement After Biomarker Approval
In addressing the audience about the various strategies to be made for improving patient involvement in biomarker development, the following key areas were identified: better communication, enhanced funding opportunities and advancing a community.
Building patient education and communication resources or portals can significantly enhance patient knowledge. For instance, by offering webinars and help videos on disease-specific biomarkers, the industry can lessen the gap between clinician and patient. An improved understanding of a disease-specific biomarker and how to incorporate it into everyday life will serve to establish a more accessible treatment process.
“When we think about the current unmet medical needs, one thing that comes to mind is this push to develop drugs in the paediatric setting.”
Similarly, supplying support grants for independent medical education to advance excellence in biomarker education will be beneficial. Finally, connecting patients to patient advocacy groups, as well as diagnostic and pharma companies, will help demystify the complexities involved in the implementation of the disease-specific biomarker and its function.
What’s Next in the Discussion Group Series?
The session concluded with a final discussion on the degree to which patients suffer from insufficient resources and community-based support. Conversation also returned to some of the key benefits of digital biomarkers for fostering patient engagement as opposed to physiological biomarkers. To watch an on-demand recording of this Discussion Group, and to discover more about the exclusive offerings from our Biomarkers Membership Community, click here.
We will continue our Discussion Group series in the new year with sessions on big data management, biomarkers in immuno-oncology, microsampling, biomarkers for disease indications such as NASH and many more. To gain exclusive access to the upcoming sessions in our Biomarkers series, click here.
*The opinions expressed in this discussion are solely the author's and do not reflect the opinions and beliefs of Bristol Myers Squibb or Amgen.
Want to stay up to date with the latest Biomarker news? Register now for Oxford Global’s flagship event, Biomarkers UK. This is a must-attend forum covering the latest trends transforming biomarker and translational research.