Non-Invasive Biomarkers: Aiding Clinical Qualification of Prognostics and Monitoring Biomarkers for Liver Cirrhosis
Presented by: Isabella Gashaw, Principal Clinical Biomarker Lead at Boehringer Ingelheim
Edited by: Ben Norris
Liver cirrhosis is an advanced stage of non-alcoholic steatohepatitis (NASH), which develops from a healthy liver with the deposit of fats. Fibrosis develops from this and worsens over time, which can in turn lead to the development of cirrhosis. As Isabella Gashaw, Principal Clinical Biomarker Lead at Boehringer Ingelheim, explained to our audience at Biomarkers UK: In-Person 2022, the disease is quite biomarker-heavy. Non-invasive biomarkers may present a means of diagnosing pre-symptomatic NASH in patients without them undergoing invasive biopsies.
- Discussion Group: Overcoming the Challenges Associated with NASH in Clinical Biomarker Development
- Treating the Untreatable: Unlocking Precision Medicine Biomarkers for NASH
- Therapeutic Efficacy in Preclinical Models for NASH
Pathologists are interested in the disease as they can investigate biopsies and stage the tissues accordingly. Boehringer Ingelheim is intending to conduct a study of 10,000 patients across a ten-year timeframe to investigate the response of patients to treatment, monitoring both patient data and patient outcomes. The study focuses on cirrhosis as the most dangerous stage of NASH. “The scar tissue leads to loss of liver function, which can be fatal,” Gashaw explained.
Issues With Liver Cirrhosis
Complications arising from cirrhosis can be severe. “This is the most dangerous part of NASH,” explained Gashaw. Scarred tissue leads to a loss of liver function and may progress to a variceal haemorrhage, which can be fatal. Liver cirrhosis may also lead to cognitive dysfunction, which is recognised as hepatic encephalopathy. Ascites is the main complicating arising from cirrhosis: in this instance, the peritoneal cavity is full of fluid which needs to be removed.
“Early diagnosis and effectiveness of care are most relevant to patients… as drug developers, we would like to have a more effective drug development timeframe.”
“Another complication arising from liver cirrhosis is hepatocellular carcinoma (HCC), where patient age and genetic disposition play an additional role,” said Gashaw. Vascular malfunctions observed in cirrhosis can also cause changes in myocardial structure and function.
Complications do not develop in all patients, as Gashaw explained. “The NASH disease itself is being diagnosed clinically mainly by non-invasive imaging techniques, but early diagnosis and effectiveness of care are most relevant to patients.” She added that different biomarker needs exist subject to the needs of patients. “As drug developers, we would like to have a more effective drug development timeframe with easier patient recruitment.”
Non-Invasive Biomarkers and the 10,000-Participant Trial
Established NASH biomarkers are applied for different purposes subject to usage. In the past they have been very well-utilised by companies such as Boehringer Ingelheim for the diagnosis of cirrhosis in non-invasive ways using imaging techniques. However, uncertainties remain regarding how patients should be represented regarding their response to treatment. “That’s a huge commitment for BI as a company,” Gashaw explained, “so we aim to conduct a study in 10,000 patients.”
Boehringer Ingelheim is currently writing a protocol for this study and would like to start conducting research by the end of 2022. The aim of the study is to segment patients and correlate biomarkers with both patient data and patient outcomes. Doing so will involve coordinating patient datasets with liver related events and cardiovascular outcomes, with the improvement of patient care in cirrhosis investigations.
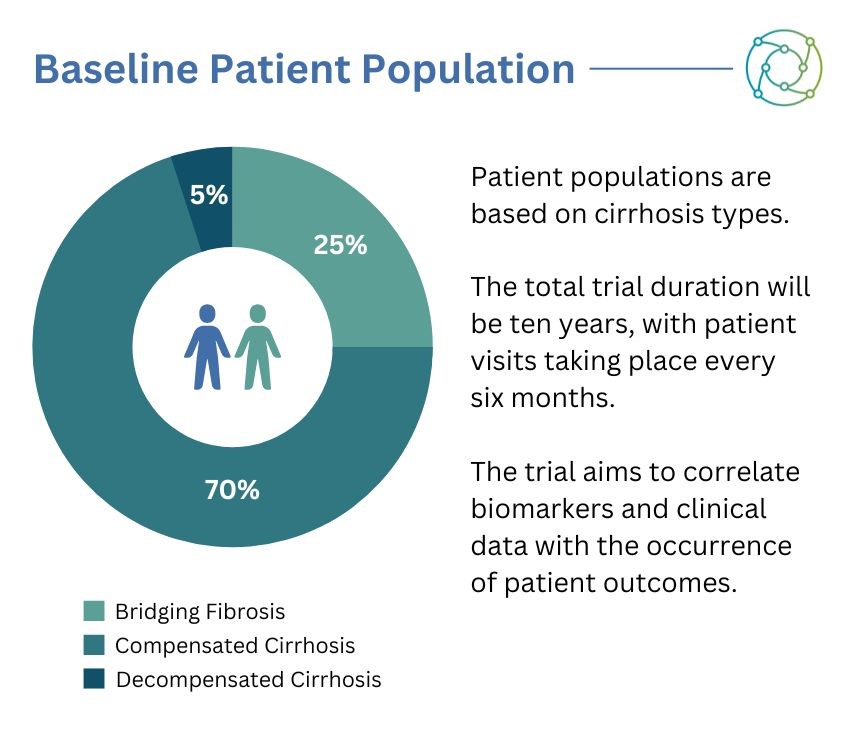
The dataset was subsequently split into three different patient cohorts. “In order to observe those events, we need to have about 7,000 patients with compensated cirrhosis,” explained Gashaw. “We will allow for about 2,500 patients with so-called bridging fibrosis, and we will allow from the beginning for 500 decompensated patients.” Gashaw added that she anticipated this number would increase over the duration of the trial as the state of the disease progressed. “We’re aiming for a global trial, so we would aim to understand patient segments across the globe,” Gashaw added.
Main Trial and Biomarker Sub-Studies
One key decision regarding the trial is that there will be no mandatory biopsies conducted at the baseline. “We really would like to include patients with severe cirrhosis – not only is it the case that they don’t like biopsies, it’s also very dangerous to conduct biopsies on patients with a low platelet count,” said Gashaw. Instead, if a historic biosample is available with the patient’s histology, this is utilised for diagnosis at the beginning of the study. The programme factors visits into routine patient care, taking place every six months.
The main study will focus on established non-invasive biomarkers, with imaging conducted by ultrasound and elastography. “This is for liver stiffness, spleen stiffness and decayed liver fat content,” added Gashaw. The ambition is that this study and associated biomarker sub-studies will deliver unprecedented insights for future for liver cirrhosis in patients.
For the main study, ultrasound imaging and transient elastography will be applied through the use of fibroscan. Disease activity-associated biomarker candidates have been identified in research consortia and other studies, with prognostic biomarkers circulating and imaging by correlation of high baseline levels with individual outcome data. On each visit, disease monitoring will be undertaken by established biomarkers to define sub-cohorts.
Study Aims and Concluding Remarks
Another sub-study which will factor into the ten-year investigation is actigraphy, which Gashaw introduced as a non-invasive method of monitoring human rest cycles. “It is known that liver cirrhotic patients have a decline in muscle mass and strength,” continued Gashaw. “This also leads to an additional reduction in physical activity and an increased fall risk, and can lead to adverse cardiovascular outcomes.”
To monitor the impact this has on patient health, a small actigraph unit, also called an actimetry sensor, is worn by a patient to monitor gross motor activity. This sensor may be a smartwatch or a similar biometric device that can be worn unobtrusively.
“As you can imagine, biomarking 10,000 patients over 10 years has a lot of challenges for the operational conduct of biomarker studies and technologies,” said Gashaw. She added that while the conceptualisation of the biomarkers used was technology agnostic, Boehringer Ingelheim had been utilising technologies used in previous trials.
Another aim was ensuring that this analysis could be carried out over an extended period and that the technology used for non-invasive biomarkers is patient-friendly. “Boehringer Ingelheim’s non-drug interventional trial in NASH cirrhosis is a patient-centric study with long-term aspirations for better patient care,” Gashaw concluded.
Want to read more about the latest research into biomarker discovery? Head over to our Biomarkers portal for up-to-date insights from the industry’s best and brightest. If you’d like to register your interest in our upcoming Biomarker Analysis Europe: In-Person conference, visit our event website.