Next-Generation Targeted Protein Degradation: Beyond the Usual E3 Ligases
During Discovery Week 2021, Ian Churcher (Chief Scientific Officer at Amphista Therapeutics Limited) gave a fascinating presentation on recent advancements and novel approaches in protein degradation. This article provides a condensed overview of the key topics covered.
History of Protein Degradation
PROTAC (proteolysis targeting chimera)-induced targeted protein degradation (TPD) has become a key area of interest for developing new medicines. As a result, it has and garnered attention from many academic institutions and pharmaceutical companies. PROTACs are small bifunctional molecules which induce ubiquitination of target proteins and employ proteasomes to mediate protein knockdown. It is not an exaggeration to say that PROTACs have opened a new door for novel drug development.
The advancement of the PROTAC field was enabled by the discovery of small-molecule E3 ligase ligands. The most commonly used are CRBN and VHL, followed by MDM2 and IAP, but this is only a tiny fraction of what is possible. The human genome encodes more than 600 varieties of E3 ubiquitin ligase, and there is still a vast area for open to further exploration and development.
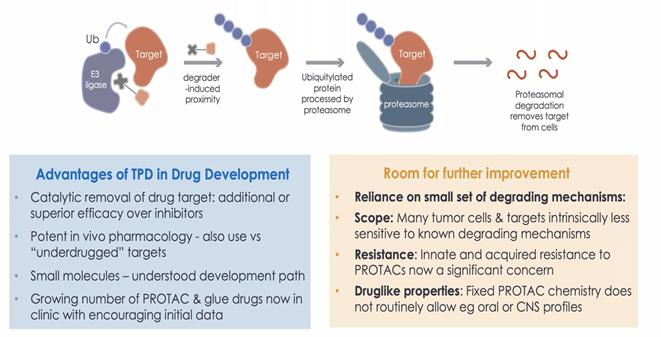
Protein degradation using E3 Ligases has had a transformational effect on drug discovery. Catalytic removal of drug target allows exceptionally efficient removal of pathogenic proteins, leading to very high potency effects, both in vitro and in vivo. There are a number of molecules using this approach now in the clinic. However, while these current therapies are working well, there is potential for further improvement.
Room for Improvement
Because current drugs are dependent on a very small set of ubiquitin E3 ligases, there are some limitations with the molecules around both the scope that those mechanisms have and the chemistry that they use. The potential for the emergence of tumour resistance is particularly concerning and, while only noted in pre-clinical systems with PROTACs so far, has the potential to lead to reduced duration of clinical efficacy.
We know many targeted oncology treatments will ultimately have problems with resistance and disease progression. But with degraders, there are more opportunities for that resistance to emerge because the degradation mechanism uses a whole range of different cellular proteins, downregulation, deletion or mutation of any one of which may allow a tumour cell to develop resistance.
The dependence of most PROTACs on a small number of VHL and CRBN-binding warheads with narrow structure-activity relationships also means that the majority of degraders have similar chemical substructures. This, in turn, gives limited chemical options for introduction of desirable chemical properties into PROTACs including oral bioavailability and the abiity to enter the CNS. Thus, further research is needed to explore the limits of the degradable area and how to chemically unlock novel ligases.
Cutting Edge Developments: Novel Targeting Methods
Amphista Therapeutics have been exploring exciting new ways to initiate protein degradation. Ian Churcher explains that “we found that there was a number of ways of inducing proximity beyond the usually E3 ligases, in fact, if you look at the ubiquitin-proteasome system, there are a very wide range of different proteins that we could use. First, of course, we have the E3 ligase substrate receptors themselves, and many people are investigating them in a lot of detail. But there’s a lot of other ligase-associated proteins. Indeed, there’s a lot of ubiquitin editing enzymes, such as deubiquitinases. And also, of course, the proteasome itself, where these proteins ultimately go to be destroyed.
We selected a range of these alternative ubiquitin-proteasome systems (UPS) proteins, where small-molecule ligands are known. We then incorporated these ligands into bifunctional molecules which may look a little like PROTACS, but, as they contain our novel warheads, we call them Amphistas. These Amphistas induce the proximity of the target to the cellular degrading machinery leading to potent degradation. We then get all the advantages of a PROTAC approach you would expect in terms of a rapid, selective degradation with long duration of action but, because of the warheads and new degrading mechanisms we’re using, we also see additional advantages across many areas including improved scope of efficacy across a broad range of cells and a good ability to introduce oral bioavailability.”
Conclusion:
Targeted protein degradation has incredible potential to provide numerous novel disease treatments and give new ways to address previously undruggable targets. Current, first-generation approaches have advanced to the clinic quickly and given a taste of what can be accomplished, and work is underway to unlock further possibilities. The targeted protein degradation field has made rapid progress. Recent developments make a strong case for further investment and research. For more on protein degradation, take a look at our upcoming events.




.png)

