Better IO Clinical Trials with ImmunoPET Imaging

At Oxford Global’s discussion group focussing on ‘Translational Immuno-Oncology and Biomarkers,’ Ian A Wilson, Chief Executive Officer at ImaginAb, outlined the “three pillars of survival for a drug in development.”
Firstly, Wilson explains that the drug needs exposure at the site of action— “does your drug get to where it needs to be in the body?” Second, the drug must ensure binding to the pharmacological target— “does your drug stick?” Finally, the drug should express a pharmacological effect— “does the drug do something once it gets there?”
These three necessary factors for a drug’s success are studied rigorously during preclinical development. However, as Wilson notes, the problem is that (especially in the IO space) the models they analyse do not always translate well when the drug moves into clinical trials. This is the need that Wilson’s company has set out to cater to, by setting up a method where clinicians can understand how the drugs they have developed work in a clinical setting.
Expansion of PET Imaging in Trials
ImaginAb has developed a new positron emission tomography (PET) agent (CD8 Immuno PET) that images immune cells. PET imaging employs radioactive isotopes as ‘tags’ on binding molecules, which can then be detected and placed in the context of a full-body image by a PET/CT scan.
Wilson says that the advantage of PET in the IO setting is that it can provide a whole-body image, as opposed to a biopsy which only samples a tiny percentage of the tumour. Furthermore, a biopsy will likely have to be repeated multiple times to track the progression of the tumour, which could be discouraging for patients as biopsy is a painful surgery.
More advantages of PET include the fact that it allows clinicians to understand the shape and dimensions of the tumour, which are often indicators of its progression. Methods like CT can also provide this information, but Wilson warned against the problem of “pseudo-progression,” where the tumour can actually expand inside as T-cells flood into it. This leads to false readings, especially when the patient’s primary treatment is immunotherapy.
ImaginAb’s ImmunoPET can determine whether a patient responds effectively to treatment in an immunotherapy context. It is also clinically translatable from the preclinical setting. It can be used to identify whether a drug in Phase I and early Phase II trials is working as predicted.
Targeting CD8 T-cells and Using PET Imaging
ImaginAb’s drug, 89Zr-Df-Crefmirlimab, uses the radioactive metal Zirconium-89 (89Zr) attached to a minibody biologic. The team decided to target CD8 T-cells because they are associated with desirable prognostic outcomes in many cancer indications: “If the tumour has lots of CD8 T-cells, [the patient] is more likely to respond to immunotherapy.”
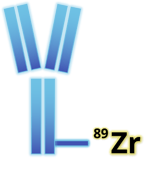
Their minibody biologic is about half the size of a full-sized antibody and has a high affinity to the CD8? chain. Its small size means it reaches the target site very quickly after injection. The isotope 89Zr has a half-life of around three days, and the product has a shelf life of six days, which is long enough to centralise manufacturing and distribution.
Although the drug is still an investigational product, “the compound is intrinsically inert,” says Wilson. The FC regions of the minibody have been removed, which makes it effectively a passive carrier; it doesn’t affect the T-cells in the body at all. Therefore, it has been deemed safe to use in clinical trials by regulatory authorities like the FDA.
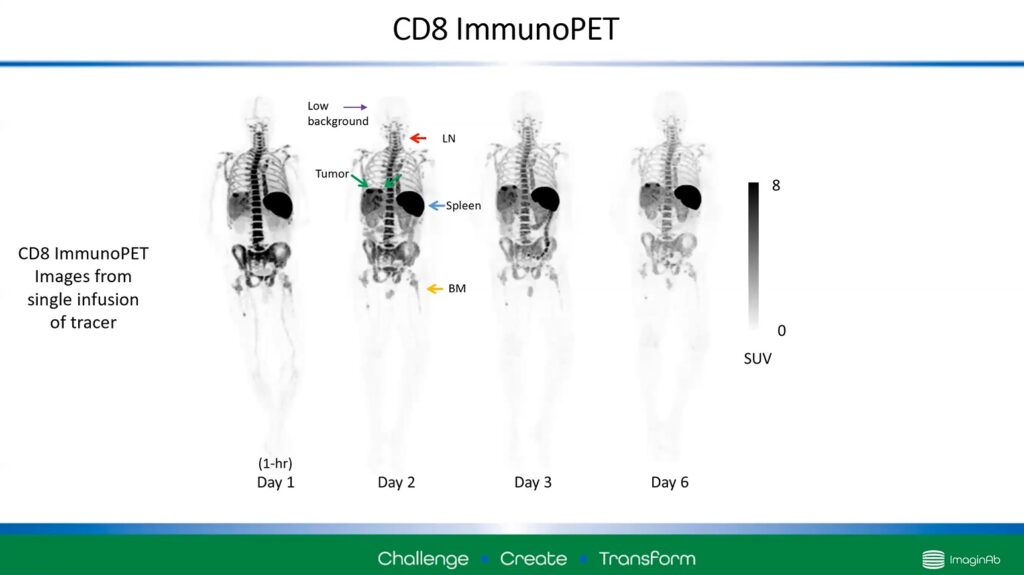
Figure 1 – Using ImmunoPET, images can display areas of strong CD8 T-cell activity. Dark regions indicate this activity.
Figure 1 shows four scans taken one hour to six days after injection. The darker areas of the image are regions in the body that are highly associated with CD8 T-cells: the spleen, lymph nodes, and bone marrow, as well as a tumour indicated with green arrows.
Three ImmunoPET Case Studies
Wilson demonstrated the advantage of CD8 ImmunoPET by considering three patients given checkpoint inhibitors (shown left to right in Figure 2).
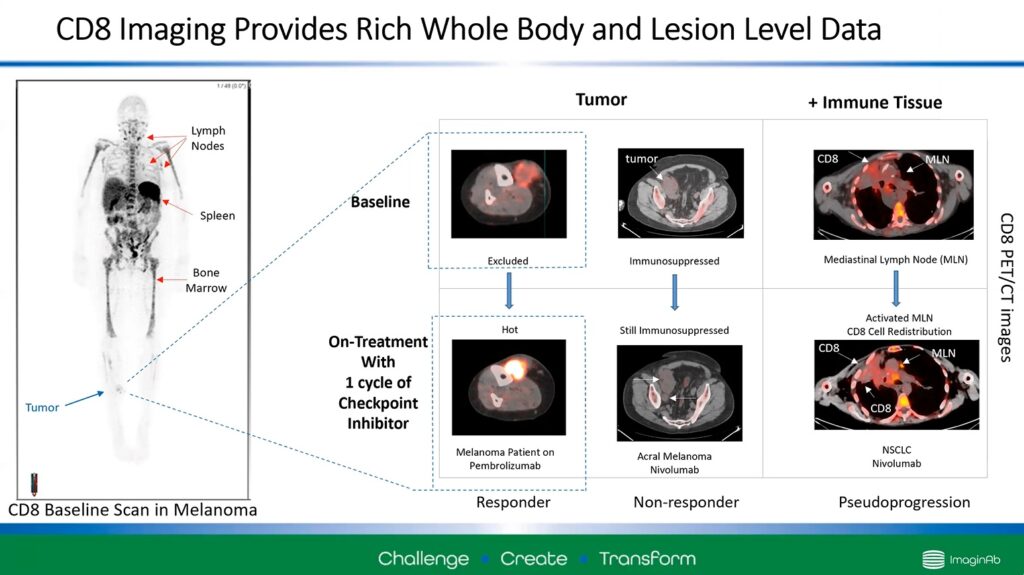
Figure 2 – Three examples of CD8 ImmunoPET use in cancer patients. Bright red spots show CD8 T-cell activity.
Wilson described a “doughnut” of CD8 activity around the tumour in the first example before treatment (top left). This indicates that the T-cells are surrounding the tumour but cannot enter it. After a cycle of checkpoint inhibitors, the PET scan clearly shows that those cells can penetrate the tumour site (bottom left). This indicates that the patient responded to treatment.
The second example (middle row) shows an immunosuppressed patient. Before treatment (top middle), there are very few CD8 cells at the tumour site. After treatment (bottom middle), the trend continues. There is still very little T-cell activity, and the tumour has increased in size, which indicates that the patient is not responding to the treatment.
Wilson’s final example demonstrates an advantage of the ImmunoPET technology. It shows no significant change in CD8 activity around the tumour site after treatment (bottom right). However, a noticeable increase in intensity at the mediastinal lymph node suggests that the therapy has redistributed the T-cells to the lymph node and not the actual tumour.
Herein, ImmunoPET can alert clinicians if the treatment is ineffective and why it may be ineffective. Wilson says that this may mean that clinicians may want to continue the treatment in this case and wait to see if there is a change in response.
How can CD8 ImmunoPET be used in clinical development?
The advantage of CD8 ImmunoPET is it allows clinicians to define the CD8 status within the tumour microenvironment and the rest of the body. This allows you to classify patients’ tumours into hot, cold, and immune-excluded categories after treatment which is highly beneficial in clinical trials.
- Perfecting Precision Medicine: The Promise of Biomarkers and Spatial Biology
- Three Leading Perspectives on Biomarker Diagnostic Discovery in Immuno-oncology
You can also stratify those patients before a clinical trial and define them into different cohorts. For example, the trial could observe treatment in patients with a high CD8 status against a cohort with low CD8 status, providing more valuable data to the trial.
The technology additionally has excellent potential for selecting the most optimal sites on which to perform different treatments, such as intertumoral therapy, radiotherapy, and biopsy. All the while, ImmunoPET can help optimise dosing, parallel and sequence multitherapy, and quick identification of early responders to treatment.
Wilson demonstrates how ImmunoPET can be an invaluable tool for conducting clinical trials for immunotherapeutic treatments. Therefore, the technology has the potential to elevate the speed and direction of clinical candidates’ translation into the clinic.
Our Preclinical Immuno-Oncology: Online conference covers key areas of interest within preclinical immuno-oncology. This interactive online conference is designed for immunotherapy experts to stay up to date with the latest models and approaches impacting their workflow. Discuss innovative approaches to preclinical development, with a focus on preventing translational failures through the development of predictive and robust preclinical models.