The Past 20 Years of RNA Therapeutics: COVID Vaccines and Beyond

Edited by Tom Cohen
From the beginning of the COVID-19 pandemic to the eventual mass-rollout of vaccination programmes across the world, we have seen mRNA go from a fairly niche therapeutic modality to a near household name.
Antonin de Fougerolles, CEO of Evox Therapeutics, has spent much of his career investigating this area. He was one of the first employees at both Alnylam Pharmaceuticals (becoming VP Research) and Moderna Therapeutics (founding CSO) and his work played a key role in the development of RNA interference and mRNA therapeutics. Giving the keynote address to Oxford Global’s RNA Therapeutics and Delivery conference, de Fougerolles explained how mRNA vaccines had not been the beginning of this field and will certainly not be the end.
Using Lipid Nanoparticles for RNA Interference
Dr. de Fougerolles began his talk by recounting the journey that his work on using lipid nanoparticles (LNPs) for RNA interference (RNAi) had taken. “We very quickly found LNPs to work in non-human primates,” said de Fougerolles, discussing an apoB-silencing DLinDMA-based LNP that he had worked on while at Alnylam.
According to de Fougerolles’ slide, the first landmark paper on LNPs demonstrated rapid, potent, dose-dependent, and durable effects. Further work published in 2010 elucidated the ways in which the company could evolve the potency of the LNP system by modifying the cationic lipid. “That’s the lipid that is responsible for engendering the endosomal escape of the cargo,” he added.
De Fougerolles explained that compared to the original 2006 paper that used a DLinDMA lipid, switching to an MC3-based lipid demonstrated a thousand-fold improvement in potency. “This was due to the improvement of the ability of the lipid nanoparticle to get the cargo out of the endosome,” said de Fougerolles. As a result, MC3 became what the company ended up using in their first approved drug: Onpattro (patisiran).
Improving RNAi using GalNAc Conjugates
While working on lipid nanoparticles, de Fougerolles’ team realised that they needed something with a better therapeutic index to be used more broadly in a range of other diseases. Alnylam, through the leadership of Mano Manoharan, started working on chemistry; how to stabilise the siRNA and direct it to specific cells in the body, looking at both the siRNA itself, its linker, and the GalNaC hepatocyte targeting ligand. Over the years, their efforts to improve the chemistry saw a thousand-fold improvement and showed dramatic clinical efficacy.
De Fougerolles group, led by Kevin Fitzgerald, developed the first PCSK9 inhibitors, which they tested with LNPs. However, they found that the duration of activity they were able to achieve was not sufficient while using LNPs. As a result, Fougerolles’ team switched their delivery method to GalNAc conjugates, which allowed them eventually to induce silencing for over six months with a single dose. That GalNAc-conjugated PCSK9 siRNA drug is now approved and marketed by Novartis as Leqvio (inclisiran).
LNPs and mRNA Delivery
Dr. de Fougerolles also recounted that when he had joined Moderna as founding CSO and among the first half dozen employees, the mRNA field was extremely small. At Moderna, the original focus was to use mRNA exclusively for therapeutics. The goal was to make an mRNA therapeutic to administer to patients, resulting in intracellular or secreted protein production.
This is what led de Fougerolles’ group at Moderna to develop and patent the first demonstration of LNP-formulated mRNA delivery in vivo. Their patent (which intravenously delivers chemically modified mRNA targeting either erythropoietin (EPO) or granulocyte stimulating factor (G-CSF)) worked across species, including in non-human primates, and showed that you could get very high levels of expression and biologic activity.
At this time in May 2012, researchers Katalin Karikó and Drew Weissman were publishing similar research: an intraperitoneal injection of EPO mRNA, with research reagent (TransIT-TKO) to increase haematocrit in mice. Dr. de Fougerolles described how this is further evidence of the potential therapeutic benefit of mRNA therapeutics.
When looking at the immune response in their experiments, the Moderna team saw that “even with modified mRNA, the activation of the innate immune response wasn’t completely abrogated.” Dr. de Fougerolles knew that this meant there was more work to do, as activating the innate immune response can lead to decreased or stopped mRNA translation.
In light of these successful studies, Moderna developed a ‘multipronged approach’. This approach would develop the design of the mRNA sequence itself, discover new improved mRNA chemistries, and continue work investigating additional LNP formulations and different routes of delivery. These three areas would all be critical in the development of mRNA therapeutics.
“We came out of that with some novel modified chemistries, including 1-methyl-pseudouridine,” de Fougerolles reported. This led to 10 to 100-fold improved protein production over pseudouridine containing mRNA and importantly, “the innate immune activation was dramatically reduced even further.”
COVID-19 and mRNA Vaccines
However, Moderna still encountered issues when repeat dosing their LNP-formulated mRNA. This meant declining protein production after three or four doses. De Fougerolles hypothesised that there must have been some level of innate immune activation when using repeated dosing.
Therefore, it was decided by de Fougerolles and Moderna to start looking at vaccine applications for the technology in 2013. “We even pitched mRNA back in 2012 as a potential pandemic preparedness treatment. This was informed in part based on my prior knowledge and experience in pandemic preparedness and on the ability to rapidly make mRNA drugs based on sequence knowledge alone,” Fougerolles added.
- Embracing The Power of RNA Therapeutics
- mRNA Manufacturing: Scalability and Process Analytical Techniques
- In Conversation With... Denis Drygin
This turned out to be a worthwhile investment of Moderna’s mRNA expertise. “Fast forward to today and we have two wonderful mRNA-based COVID-19 vaccines both using LNP technology and 1-methyl-pseudouridine chemistry,” said de Fougerolles. Since 2012, there have been seven key Moderna patents covering mRNA vaccine technology, including two by de Fougerolles and Guild that broadly cover mRNA chemistry and LNP formulations.
“mRNA is proven for vaccine applications, though its broad use in therapeutic contexts remains to be determined,” de Fougerolles noted. “There are some therapeutic programs, but they are still in early clinical development”.
New and Improved Modalities for RNA Delivery: Exosomes
Dr. de Fougerolles commented “that there are multiple approaches currently being pursued with a view to improving RNAi and mRNA delivery. Those include new chemistries such as conjugates, transferrin receptor conjugates, and the extrahepatic targeting of LNPs using new ligands and lipids.”
Furthermore, de Fougerolles is excited about the prospect of using exosomes to deliver RNA molecules. Evox Therapeutics, de Fougerolles’ current company, are using exosomes because this is the body’s natural way to deliver biologic and RNA molecules. Exosomes are similar in size to LNPs, but they don’t activate the innate immune response. As a result, they able to deliver cargo safely, effectively, and repeatedly.
“At Evox, we have been able to understand the signals that cells use to put molecules into exosomes, and we use that to deliver our own drugs,” said de Fougerolles, pointing towards the modalities that are available for exosome delivery (Figure. 1).
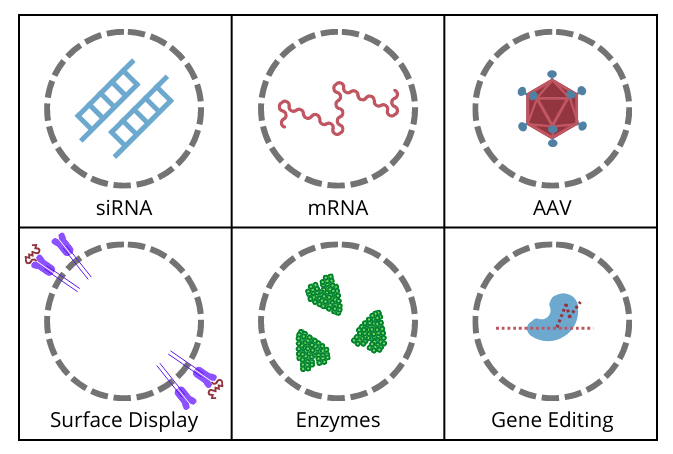
Figure. 1 – Exosome delivery - drug modalities
Dr. de Fougerolles wrapped up the talk by explaining Evox’s DeliverEXTM platform. They use engineered exosomes as a modality-agnostic enabler of precision therapeutics. This is what de Fougerolles sees as the next generation of RNA delivery method. The advantage of this method lies in using the natural capabilities of exosomes for truly targeted delivery.
Join leaders, experts, and researchers at Formulation & Delivery US: In-Person, connecting global pharma, biotech, and academia for high-level discussions on the latest innovations for biopharmaceutical development.