Sangamo Therapeutics Engineers a State-Of-The-Art Capsid, Revolutionising Drug Delivery

The blood-brain barrier (BBB) regulates what enters and exits the brain. It is crucial since it ensures the brain accesses key nutrients while protecting it from pathogens. In the capillaries, the tightly wedged endothelial cells line in interior vessels form extensive narrow junctions. The gap only allows passive diffusion on lipid-soluble drugs at a molecular weight lower than 400 – 600 Da.
As a result of its structure, the BBB blocks promising therapeutics preventing over 98% of small-molecule drugs and all biologics from accessing the brain.
To overcome this significant obstacle, pharmaceutical companies have focused research efforts on developing capsids. A capsid is the protein shell of a virus, enclosing genetic material. Both AAV9 and AAV rh10 are current gold standard capsids known to cross the BBB and are most frequently used for delivery into the central nervous system (CNS). However, AAV9 and AAV rh10 still have limited BBB penetration and CNS transduction.
Sangamo Therapeutics recently announced data from their newly engineered adeno-associated virus (AAV) capsid STAC-BBB has outperformed the gold standard capsid AAV9 in terms of transgene expression. According to a recent press release, STAC-BBB has demonstrated exceptional transgene expression levels that are 700 times higher than those observed with the gold standard capsid AAV9.
STAC-BBB was engineered using Sangamo's SIFTER (Selecting In vivo For Transduction and Expression of RNA) platform. The SIFTER platform screens tens of millions of individual capsids. Using rounds of in vivo selection, the platform identifies capsids that can move into the target tissue and express the therapeutic payload.
Related:
- Exploring Ways of Crossing the Blood-Brain Barrier
- The Utility of Nanoparticles in Tissue-Targeted Drug Delivery
- From LNPs to AAVs: Overcoming RNA Delivery Challenges
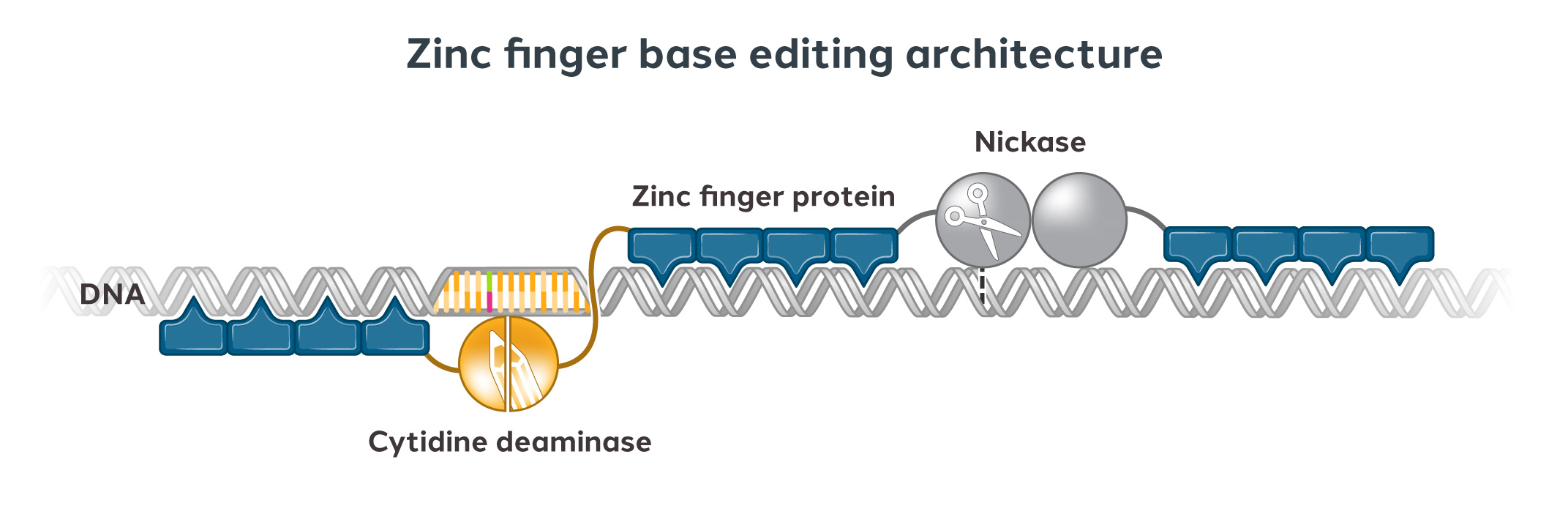
Zinc finger proteins were selected as the therapeutic payload because they are compact and can be easily packaged into delivery vectors. This allows for great genome accessibility alongside the enhanced ability for cellular delivery. The SIFTER-generated AAV capsids have demonstrated better delivery to the CNS in comparison to conventional AAVs.
Sandy Macrae, CEO of Sangamo Therapeutics, expressed an optimistic outlook, she stated: "We are extremely encouraged that STAC-BBB, a potentially game-changing capsid variant demonstrated results that outperformed other known neurotropic capsid variants achieving widespread brain delivery."
Furthermore, preclinical data from a non-human primate study demonstrated that intravenous delivery of the STAC-BBB capsid achieved widespread brain delivery and produced desired de-targeting of the liver. There were no significant treatment-related pathological findings in brain, spinal cord, or peripheral tissues from subjects in the NHP studies, which indicated that the capsid has a good safety profile. The project also showed that STAC-BBB displayed potent repression of disease-related genes, indicating a transformative impact on treating prion diseases and tauopathies.
Figure 1 – Sangamo Therapeutics's Zinc Finger Technology
Sangamo plans to leverage its capsid technology by implementing STAC-BBB into its neurology pipeline and the company has outlined plans to pursue Investigational New Drug (IND) submissions and clinical trial applications for several neurology programs by the end of 2025.
While the results of this study show promise, it is still in the preliminary stages. How STAC-BBB performs in clinical trials will be a true testament as to whether this is a reliable method of drug delivery for treating neurological conditions. Furthermore, it is unlikely that a one-size-fits-all approach will work, it is probable that scientists will need to develop different delivery solutions for different therapeutic indications. It is therefore crucial to build on and improve existing sets of capsids.
Overall, this case study emphasizes that capsids that display higher levels of delivery efficiency and specificity for target tissues set the stage for safe and effective genomic medicines to potentially treat complex yet widespread neurological disorders with high unmet needs. Given that the progress of neurological medicines has been slowed by the inability to achieve widespread CNS delivery, Sangamo's capsid could unlock solutions to addressing devastating neurological disorders.