Patient First: The Necessity of Patient-Centric Product Design for Enhanced Pulmonary Drug Delivery

Presented by: Nélio Drumond, Senior Principal Scientist of Manufacturing Sciences, Takeda Pharmaceuticals
Edited by: Cara Digby-Patel
How can we expect a patient’s use of a product to be as effective as possible when the product design process fails to consider the patient’s needs and opinions? This is precisely the question that Nélio Drumond, Senior Principal Scientist of Manufacturing Sciences at Takeda Pharmaceuticals, sought to address when speaking at Oxford Global’s Inhalation & Respiratory Drug Delivery Congress UK in May 2022. Drumond talked us through the importance of a patient-centric approach to product design when developing effective inhalation devices for enhanced pulmonary drug delivery.
- Challenges in the Formulation of Biologics: Protein Aggregation, Safety, and Technology
- Evaluating Closed System Drug-Transfer Devices
- Handle with Care: Continuous Delivery of Fragile Molecules
Patient-Centric Drug Product Design in Healthcare Provision
Patient-centric drug product design is the process of identifying the comprehensive needs of the target population and utilising this knowledge to design appropriate drug products that can be intuitively used and easily administered by patients. The therapeutic outcome will be more beneficial when the patient is at the heart of the design process because the healthcare provision will be tailored to meet the patient’s needs.
In order to implement the patient-centric model more effectively, the pharmaceutical industry must rethink its business model. Fundamentally, pharma pipelines need to change their focus to place patient needs over profit motives. Moreover, Drumond highlighted that patients in clinical trials should stop being treated purely as subjects for data collection and be viewed instead as partners working together with different stakeholders towards the same goal.
Why Is a Patient-Centric Approach Needed for Pulmonary Drug Delivery?
A wide range of devices with different mechanisms are currently available for drug delivery to the lungs, including soft mist inhalers (SMIs), metered dose inhalers (MDIs) and dry powder inhalers (DPIs) with capsule/blister/reservoir mechanisms. This variety makes it harder for patients to use them correctly and therefore ensure that the prescribed therapy is effective
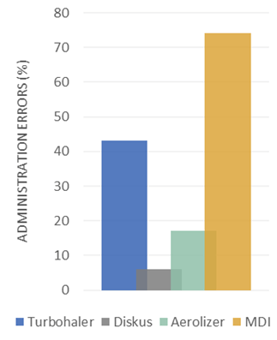
Indeed, certain mechanisms have a far higher rate of administration errors; MDIs, for example, have a greater chance of error because these require patient synchronization between device activation and patient inspiration (see Figure 1). Moreover, a change in DPI mechanism can further increase the risk of administrative errors when insufficient training is given, simply because the device is unfamiliar to the patient (see Figure 2).
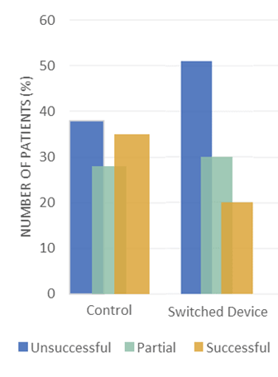
There is often a presumption that patients will understand how to use a device just by looking at it. Citing a study from April 2022, Drumond noted that inhaler technique is often not adequately assessed and reported during clinical trials. This reflects an apparent failure on the part of those conducting clinical trials to ensure that patients utilising the inhalation device do in fact know how to use it correctly. It also suggests that the actual usability of new devices is often not considered.
If a patient-centric approach is not implemented during the development stages for drug delivery mechanisms, it frequently results in a substantially lower efficacy rate. Thus, Drumond concluded that patient-centricity is “not a trend but a need”, as patients need user-friendly and high-performing devices. The best way to ensure that new devices meet these criteria is by developing them with patients.
Instances of Successful Patient-Focused Approaches
A handful of cases already illustrate the positive impact of a patient-focused design process. Trudell Medical has established a patient-centric inhalation device design process proposal which consistently incorporates the patient into each phase (see Figure 3).
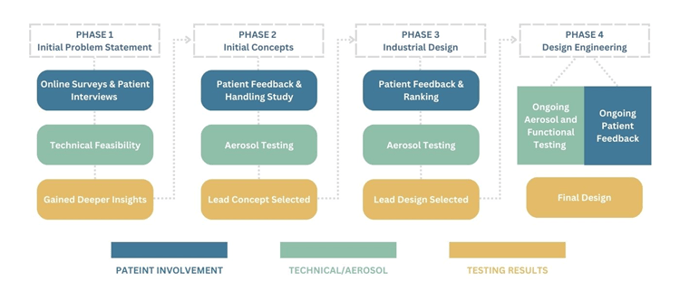
Drumond highlighted how the efficacy of this process can be observed in Trudell Medical’s development of a new portable spacer (VHC). An initial survey was taken to gauge the primary areas where patients wanted to see improvements; in particular, patients flagged that they wanted more portable and discreet devices. Developers then designed different prototypes specifically designed with these needs in mind.
By continually checking in with patients throughout the design process, developers guaranteed that the final design would meet all the patients’ needs raised by the initial survey. The patient-centric approach also rendered the redesign a far more effective means of drug administration, as there was a reduction in patient dependency during device handling and inhalation process.
The redesign of the Respimat Platform by Boehringer Ingelheim is another exemplary instance of a patient-centric approach for device optimisation. Though the older design was already recognised as easy to use by the Arthritis Foundation, the company still chose to enact a redesign based on user feedback to improve the handling and usability of the device.
Patients in clinical trials should stop being treated purely as subjects for data collection and be viewed instead as partners working together with different stakeholders towards the same goal.
The outcome was a design optimisation that delivered all the key aspects that patients wanted: easier cartridge insertion and replacement processes, improved readability of dose indicator, easy and intuitive base detachment, and bigger size to help with grip. Overall, Drumond noted that responding to patient requirements and consistently incorporating patient feedback into the redesign led to better device performance and increased convenience, ultimately resulting in more effective drug delivery.
Drumond closed by touching on where he sees the future of the patient-centric approach heading. In particular, he expects to see the integration of AI and digital healthcare in the production of inhalation devices. This would allow for an easier exchange of information between patient and provider. The outcome would be a more straightforward, streamlined assessment of whether pulmonary drugs are being administered correctly and, if not, it will result in a faster implementation of changes in device design.
Join leaders, experts, and researchers at Formulation & Delivery US: In-Person, connecting global pharma, biotech, and academia for high-level discussions on the latest innovations for biopharmaceutical development.







