Handle with Care: Continuous Delivery of Fragile Molecules
Edited by Tom Cohen
At Formulation & Delivery UK 2022, Oxford Global was delighted to host a talk presented by Dr Joel Richard, Chief Scientific Officer at MedinCell. Richard’s talk was titled Long Acting Injectables of Fragile Drugs: Challenges and Opportunities for New Delivery Technologies and outlined MedinCell’s achievements in developing a drug delivery platform for fragile drugs.
- The Past 20 Years of RNA Therapeutics: COVID Vaccines and Beyond
- Realising The Potential of Nanomedicine – Formulation & Delivery Approaches
- Technologies Advancing Drug Delivery Through the Blood-Brain Barrier
MedinCell develops new drugs using proprietary technology: block copolymers. Richard says that their platform has been successful so far in bringing new products into the clinic. Now, they are investigating the opportunity to use this technology for fragile hydrophilic molecules like biologics and peptides.
Richard laid down the challenges for delivering what he called “fragile molecules” – both proteins and peptides - due to their poor stability in formulation processes, their enzymatic degradation and poor absorption across epithelial membranes, as well as their short plasma half-life. As such, the molecules’ half-lives need to be increased to deliver longer exposure, enhancing their safety and efficacy balance. Furthermore, Richard explained that fragile molecules have a difficulty crossing membranes due to their low permeability related to high molecular weights. A solution to this that MedinCell are developing is long acting injectables.
“Long acting injectables can continuously deliver drugs systemically and/or locally,” said Richard. This delivery method allows for the controlled and customised release of therapeutic agents. As a result, they can keep the concentration of released drugs within the therapeutic window for extended periods of time. “There is also the possibility for local delivery, for instance to the joint,” added Richard.
What Has Been Successful So Far?
Richard discussed three types of delivery solutions for fragile molecules that have been investigated so far, some products being more successful than others. The solutions that Richard mentioned utilized nano or microparticles, solid implants, and more recently liquid in situ forming implant formulations. “The question is: what systems have been successful so far at delivering fragile molecules?” asked Richard.
“For peptides, we have seen that they have been quite successful while still needing improvement. But for biologics, no novel delivery system has really been successful so far – none based on these technologies available on the market at this present time.”
Researchers have found that PGLA microparticle delivery systems have proven successful for delivering peptides. Dosing cycles are typically between one and three months, requiring an intramuscular injection – although more recently, subcutaneous versions have been developed. Solid implants have also found success. These implants can be biodegradable, or non-biodegradable versions can be adapted for longer periods of release – “typically six months to one year,” said Richard.
Finally, a new generation of implants has since been developed and put on the market: in situ forming biodegradable implants, such as the Eligard® system from pharma company Tolmar. Eligard® has been used for the subcutaneous continuous delivery of LHRH for prostate cancer treatment. The platform combines ease of administration with long release duration (one to six months) of fragile molecules such as proteins and peptides.
The technology is not without limitations and challenges, however. Richard pointed out that the solution is not stable as a liquid formulation in the long term. “So, there is a need for a complicated reconstitution procedure with four or five steps using a push-pull mixing system,” explained Richard.
MedinCell’s BEPO Technology
MedinCell’s most recent development in this field is in developing the second generation of in situ forming implants from ready-to-use formulations. This new technology addresses the challenges of the first generation, making it small, stable, and ready-to-inject.
“This brings us onto BEPO developed by MedinCell,” said Richard. MedinCell’s technology “combines API for pharmacological activity, solvent for injectability, and copolymers for controlled release.” The copolymers that this technology is based on are a combination of diblock (DB) copolymers and triblock (TB) copolymers (see figure. 1).
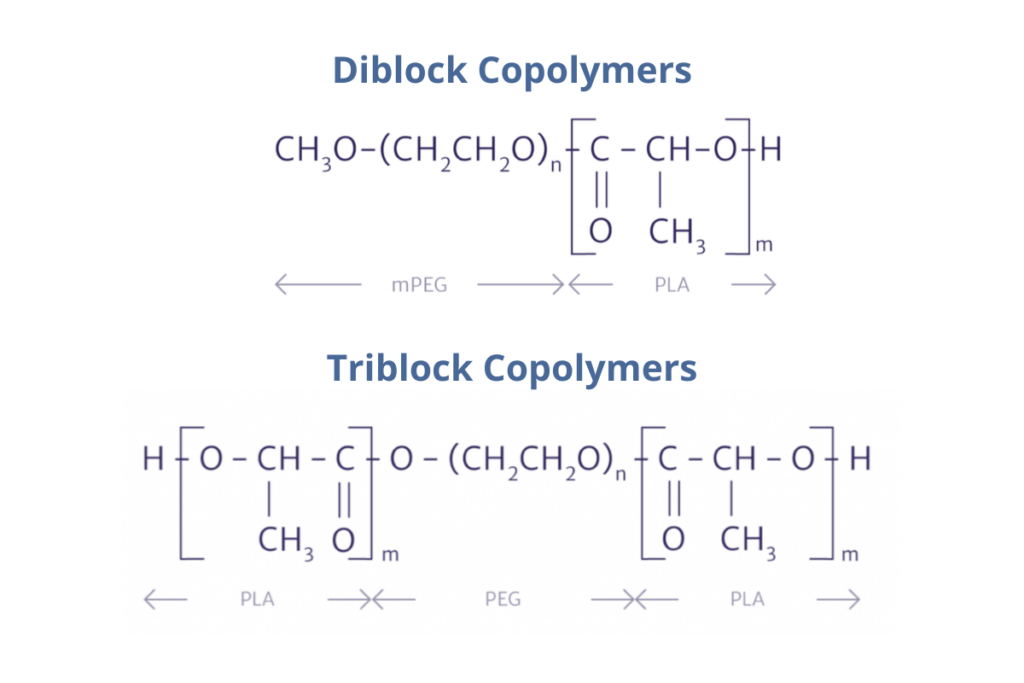
Richard said that this allows for “great flexibility,” enabling the modification of a variety of attributes of the product. For example, duration of action, viscosity, stability, and solvent and API form can all be changed. “But the most interesting part is that you can play around with the kind of polymer “lego,” based on the triblock and diblock.” By selecting the appropriate TB to DB ratios and molecular weights, it is possible to adjust the profile of release – making it an almost modular system.
“When you prepare long-acting injectable peptide formulations with biodegradable polymers, the key challenge is the stability,” Richard explained. “More particularly, the interaction between the peptide and the polymer through covalent binding: an acylation occurring on the N-terminal group of the peptide.”
Toward Successful Formulations
Demonstrating this, Richard gave the example of an octreotide formulation. Looking at the in vitro release profile, Richard pointed out spots showing that a part of the peptide was acylated – “this can affect the bioactivity of your product.”
Using the BEPO formulation, Richard showed that octreotide acylation can be controlled. “For instance, by the addition of divalent cations such as Ca2+ and Mn2+, you can produce a very strong decrease in acylation.” Furthermore, Richard described using hydrophobic peptide counter-ions (such as pamoate, DDS) to invoke the same decrease. “So, it is possible to stabilise the peptide and avoid the interactions with the polymer.”
Interestingly, you are also able to control the Cmax, which is a factor that may be related to some adverse events. “By choosing the DB composition, you can strongly decrease the Cmax and adjust the PK profile of the formulation,” said Richard, adding that this can lead to a prolonged half-life. By tweaking the formulation, MedinCell were able to increase the dose duration to one month and above. Further adjustments from the team led to the realisation that modifying the TB composition can significantly improve the bioavailability of a peptide.
Join leaders, experts, and researchers at Formulation & Delivery US: In-Person, connecting global pharma, biotech, and academia for high-level discussions on the latest innovations for biopharmaceutical development.






