CHO Cells and Engineering Cell Line Approaches: Addressing Challenges in Manufacturing
Optimising for cell line expression and productivity has been a keenly followed pursuit in the gene editing community for the past few decades. Immortal cell lines, such as those derived from HeLa cells or CHO cells, are often used in research in place of primary cells. Cell populations are maintained in culture for an extended period, retaining the stability of certain phenotypes and their functions.
Some of the most popular areas of discussion around cell line development at present focus on shortening the workflow, with the improvement of analytical power. There has been debate over whether an upper ceiling has been reached in terms of the volume of product that can be synthesised through conventional manufacturing procedures.
- How will RWE be integrated into cell and gene therapy trials?
- Overcoming regulatory hurdles associated with GMPs
- NK cells and the potential for addressing unmet clinical needs
Oxford Global's Cell 2022 event explored some of the major challenges concerning cell line manufacture, including issues surrounding genetic instability and future mutations. Chinese hamster ovary (CHO) cells have enjoyed a long stint in the sun as the industry's cell line platform of choice. But with constraints in output prompting researchers to look for alternatives, is there a new challenger on the horizon?
Cell Line Engineering Backgrounds; CHO Cells and Heterogeneity
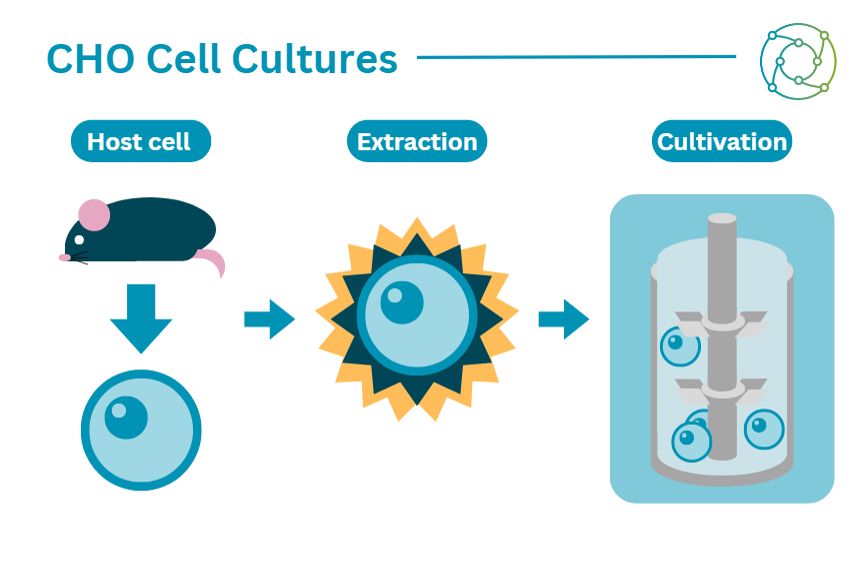
CHO cells have been the industry standard for cell line manufacture for decades. They tend to be the host of choice to produce recombinant protein therapeutics, as they are highly efficient in terms of yield. The proteins produced in CHO cell cultures are like those produced in humans, and they are easily growable in large-scale structures. However, there are disadvantages to using CHO cells - such as low productivity and cell density - which have spurred the search for other reproducible proteins.
Paula Meleady, Assistant Professor in the School of Biotechnology at Dublin City University, has done a significant amount of proteomic work on CHO cells. A key focus for her is interpreting proteomic data to investigate how the broader cell line could be improved. “With genetic instabilities and clonal instabilities, one of the main challenges is trying to make sense of the data that we're generating,” said Meleady. Heterogeneity is a particularly challenging hurdle in negotiating CHO cell engineering approaches, due to the sheer variety of mutations that may be expressed in cellular material that is functionally identical.
“One thing we're quite interested in - if you're really pushing the cell - is the distress it puts on the cells themselves and all the subsequent problems that causes, like with apoptosis or other types of systems,” continued Meleady. In her opinion, this space can be pushed for further exploration and investigation. “I think it's such a complex thing with the cell itself.”
Modelling Prediction in Cell Line Engineering and Manufacture
Having confidence in the predictiveness of small-scale data is integral to monitoring cell line behaviour. With higher degrees of accuracy, informed decisions can be made on the best-practice approach to cell line engineering and development. In the cell manufacture process, there are steps that come before understanding and interpreting what specific data means.
If researchers end up with thousands of clones to screen, this is a sub-optimal situation - the best approach is to narrow down soon as possible. Doing so will help to save on both time and expenses later in the manufacturing process. Another key emphasis in laying out strategies for cell line manufacture is selecting the correct clone for gene transferability. When dealing with a large volume of data, the best practice approach is to integrate it into one system to make sure the process informs an educated decision.
Inflection Points for Cellular Manufacture
If a productivity ceiling of a few grams per litre of titre has been reached in most CHO cell lines, the next focus in synthetic biology may instead be in manufacturing proteins that are difficult to express. Once manufacturers and suppliers surpass the volumetric titre of product, the costs of the entire manufacturing process shift away from cell culture engineering - the inflection point becomes something else. With this shift enforced and magnified, new therapies could be brought to the clinic which otherwise would not be available.
Hypothetically speaking then, do manufacturers need cells to develop their product? As Professor of Biological Systems Engineering at Imperial College London, Cleo Kontoravdi has significant experience with cellular systems and their cell-free counterparts. “Some cell-free engineering strategies are great success stories,” she said, “and are scalable and can reach very high titres.” The comparison of traditional clonal cell lines and CHO cell lines versus cell-free systems is an emerging area of conversation for those in manufacturing.
Cell biology should not be overstated as a factor for bottlenecks in traditional CHO cell lines for making difficult-to-express productions. “I think there's a lot of work that needs to be done towards understanding why some antibodies are difficult to express and produce in CHO cells,” said Meleady. Investigating secretory-type pathways offers one means of assessing things further, as does mapping out antibody configurations.
Alternate Solutions for Improving Cell Line Productivity
While there is no single quick fix for improving productivity, there are a range of different approaches which could help cell line output. One example for monitoring the upscaling of manufacturing processes would be digital twins, which would be useful for predicting the behaviour of certain developments and improving an understanding of the data derived.
Technology used in drug development is broadly driven by biological necessity rather than engineering acumen. Unlike in other disciplines where solutions are engineered from the ground up, manufacturing tends to be driven by the nuanced requirements of the living material. Some suggestions for improving modelling provision from those in the industry include fostering closer links between teams involved in industrial and academic research, along with targeted research and development.
Regulatory approval is, as ever, a factor to bear in mind during the later phases of clinical trials. Developers need to convince the FDA their process works the same way each time, with a consistent product output. Keeping this in mind at the start of setup is another box to check, but it can also help avoid hamstringing an otherwise successful cell line engineering project at the final hurdle.
Want to receive the latest industry announcements on cell manufacture and development? Sign up for our Cell series newsletter to get up-to-date news each month. If you'd like to know more about our upcoming 3D Cell Culture conference, visit our event website to download an agenda and register your interest.