Beating the System with Advanced High Throughput and Real-Time Analytics for Biopharmaceutical Manufacturing

High throughput analysis is a form of measurement science that enables accelerated catalyst and enzyme screening for synthetic chemistry. The technique can improve the quality of analytical processes and addresses the complexity of scientific data at a reduced cost.
By implementing high throughput analytics and other emerging technologies such as real-time release testing (RTRT) and computerised automation, biomanufacturers can work to improve manufacturing processes on the shop floor as well as the quality control lab. These advanced technologies can also apply to real-time monitoring throughout the life cycle of drug substance and Drug product manufacturing life cycle.
Why Is There a Need for Advanced High Throughput Analytics in Biopharmaceutical Manufacturing?
Biopharmaceutical manufacturing is a highly complex process with an equally intricate commercialisation life cycle (see figure. 1). “Maintaining the product and process quality during manufacturing of biopharmaceutical including biologics therapies is the priority,” Udayanath Aich, Director at Bristol Myers Squibb, explained. “The maintenance of clinical safety and efficacy in order to sustain the product and delivery a similar or better quality, is highly important.”
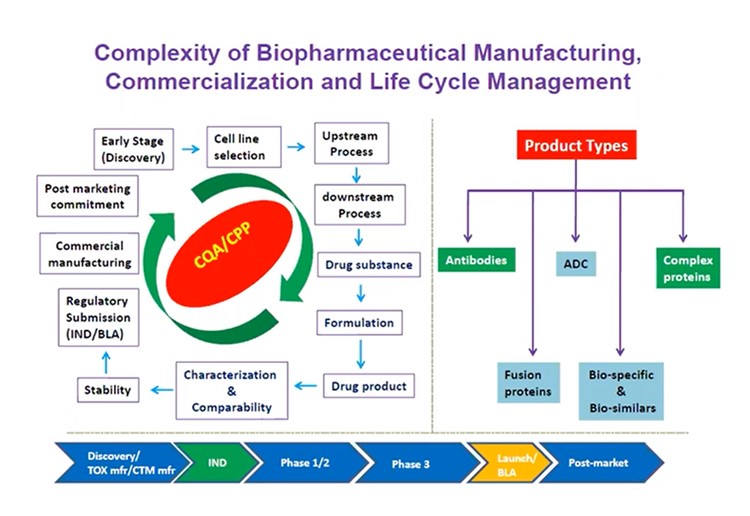
Aich said that when it comes to conducting analytical characterisation and comparability studies, “biopharmaceutical manufacturers need to make sure they are aligned on a clear processing direction.” Any confusion or inconsistency can lead to an analytic nightmare later down the line, thus harming the final drug product's success.
Certain biologics product types can make the manufacturing life cycle particularly challenging. For instance, atypical antibody fusions, antibody-drug conjugates, and bi and tri-specific complex proteins and biologics often present complexity in terms of product quality analysis.
Motivation for Real-Time Monitoring
Real-time monitoring is technique that provides an accurate return of information relating to the current state of queues and channels within a queue management system. There are many advantages and motivations behind real-time monitoring, and according to Aich “it is a strategy that reflects the rapid evolution of analytic advancement.”
- Advancing Stem Cell Manufacturing
- Potency Assays for ?-Thalassemia and Sickle Cell Disease Gene Therapy Drug Products
- Combinatorial Culture Technology for Stem Cell Therapy Applications
A process intensification strategy, real-time monitoring facilitates improved customer-based utility and advanced manufacturing. “It provides a clear set of customer prerogatives which includes a reduction of cost with improved quality,” Aich said. In particular, the technique harnesses advanced manufacturing of biologics by performing both batch-fed processing and perfusion culture to harvest cell culture fluids suitable for downstream purification.
Real-time monitoring addresses the current manufacturing demand for the increased speed of release testing. This, as Aich explained, “can establish a critical control point for benchmarking an enhanced control strategy to in turn save significant processing time.” Finally, in moving away from end product testing to real-time release testing, the industry can facilitate a continuous manufacturing process for the future.
Emerging Analytics for Process Control and Product Release
Aich also discussed how the use of emerging analytics provides a springboard for quality determination. Emerging analytics refers to the analysis of products during all stages of manufacturing to generate data based on critical control points.
With real-time process knowledge, monitoring, and control, paving the way for the future, global adoptability will soon become a reality for the future of high throughput analytics.
Various process parameters must be introduced to determine and control overall product quality. For upstream cell cultures, there are four predominant parameter measurements: nutrients, metabolites, reactors, and cell parameters.
The Evolution of Advanced Analytics: Forecasting the Future
Whilst traditional methods of analysis such as chromatographic and mass spectrometric tools are easy to adopt due to the prior existence of life cycle analytics, they are invasive and time-consuming. Instead, non-invasive advances such as next-generation spectroscopy and chemometric tools are fast becoming the trend for biologics biomanufacturing.
The hope is that these technologies of tomorrow will streamline data analysis and surveillance of biopharmaceutical manufacturing including biologics therapy manufacturing for the better. With real-time process knowledge, monitoring, and control, paving the way for the future, global adoptability will soon become a reality for the future of high throughput analytics.
Want to find out more about the latest cell therapy news? Register now for our Cell Therapy Analytics Symposium to find out more about novel characterisation techniques &robust analytical tools to ensure safe, quality therapeutic cell products are advanced through to the clinic.







