Advanced and Predictive 3D Cell Culture Models
In November 2022, Oxford Global's Cell series was delighted to host a panel discussion focusing on Advanced and Predictive 3D Cell Culture Models. The discussion covered several key topics, including how these models are designed and their uses, the reproducibility of 3D models such as organoids or organ-on-a-chip models, and their viability.
The conversation was led by Elodie Vandenhaute, Project Director at HCS Pharma. Notable attendees were representatives from Merck, Takeda Pharmaceuticals, Merck Animal Healthcare, and the Medical University of Graz.
What Are 3D Cell Culture Models, and Why Are They Used?
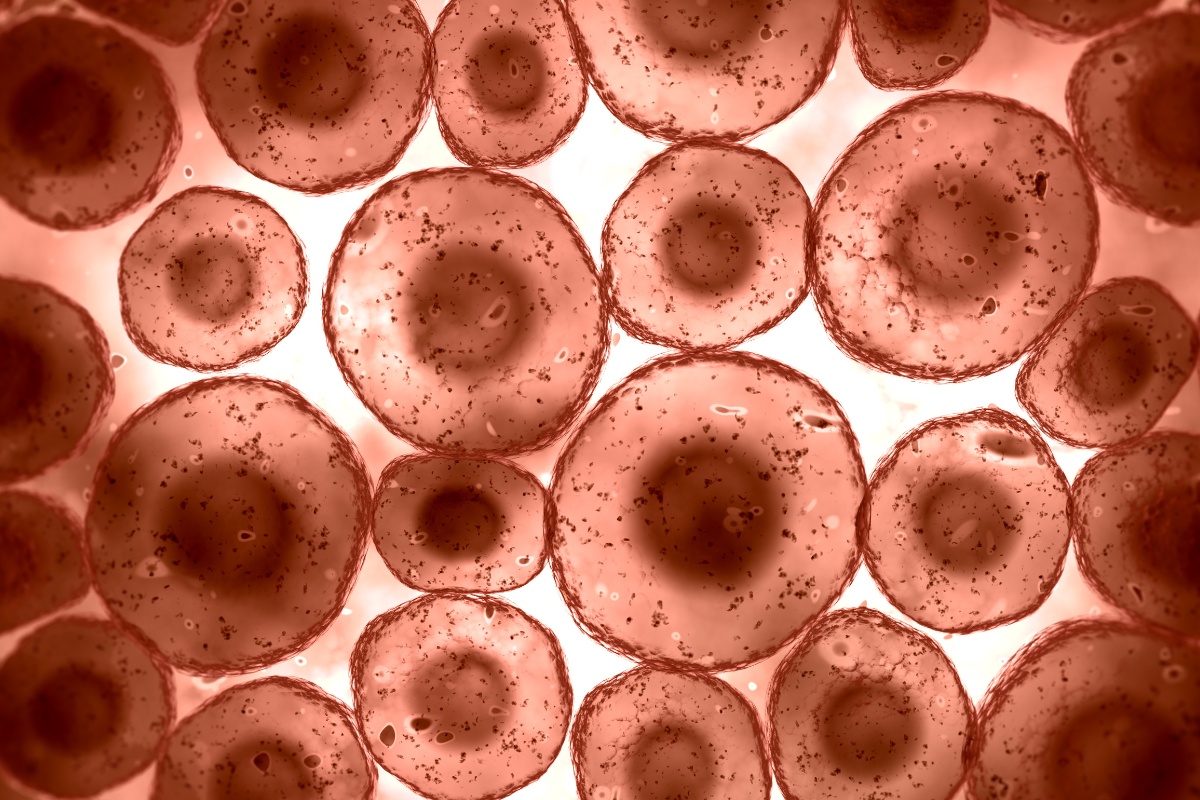
The discussion began with an overview of what is generally understood by 3D cell culture models. The fastest-growing field within 3D cell models is organoid development. Organoids are a group of cells either in mono or coculture, depending on what's put inside of them.
Organoids can be grown in ultra-low attachment or in hanging drop systems, where the cells are not embedded in a matrix. However, the primary focus of this discussion was organoids that are grown within a matrix, where they build 3D structures, or they are kept in a 3D environment where they can grow into little spheres, or spheroids, which are typically not significantly more complex than other spherical structures.
Vandenhaute was keen to draw the audience's attention to organ-on-a-chip models, a relatively new technology that can also be considered 3D. Organ-on-a-chip models are complex cocultures of cells in very small vessels which can be placed directly onto the microscope. They can also be used in metabolic experiments, or to put different types of cells or tissues into contact.
The discussion then turned to the question of why such models are used. To better understand therapeutic mechanisms, researchers need a clear understanding of tissue complexity. This comes from knowledge of the cell types within tissues, the matrices around cells, and the vessels in the tissues.
Vandenhaute stressed the need for representative models in this process; whilst mice models are helpful to an extent, the mechanisms are generally too far removed from those found in human tissue to accurately represent the effects of drugs or the mechanisms at stake with different diseases.
- Video Q&A – In Conversation With…Stefan Przyborski, Professor, Durham University
- Understanding the Capabilities of 3D Bioprinting for Cell and Gene Therapy
- Apical-Out Organoids: A Novel Strategy to Control the Polarity of Organoids Closing
This is where 3D cell culture models come in. Researchers can improve their predictions for drugs' functioning within human tissues by creating models grown from human cells. Humanised models are a far more faithful reflection of human tissue complexity.
Moreover, 3D cell culture models mean that cells are far more durable and can be kept for much longer, making the research process much more manageable whilst also providing a more accurate prediction of human outcomes.
Creating 3D Cell Models
Audience members were keen to understand the best approach to designing 3D models. Ultimately, organoid design depends largely on the intended use and the question it will answer.
Depending on what the model is being designed for, the origin of the cells used is very important. If primary cells are cultured, it is possible to grow models which combine different cell types. Alternatively, induced pluripotent stem cells (iPS) can be differentiated and derived into complex organoids.
Vandenhaute noted that at HCS, iPS have been used to produce liver organoids in a dedicated 3D matrix to research metabolic diseases. Using these cells was incredibly beneficial as they produced well-differentiated organoids, which contained a range of liver cells, including hepatocytes and stellate cells. According to Vandenhaute, the extracellular matrix is an important parameter to take into account when designing relevant 3D cell culture models.
When creating organoids for oncology studies, it is most likely that patient samples will be used to grow the models, meaning that there is naturally variation between samples. Moreover, when these cells are put in culture and observed, a wide range of organoids emerge. Therefore, knowing which organoids will be representative and the most beneficial to work with is essential. Considering how many organoids will be needed to provide enough data to reach a valuable conclusion is also a significant factor when designing 3D cell models.
Organoid Standardisation and Reproducibility
Following this, one audience member asked if there was any way that organoid models could be standardised. Vandenhaute acknowledged that there is a general issue with reproducibility, as often it is hard to produce uniform models consistently.
One method to standardise cell culture is by starting with cells which have been isolated into individual cells using a gentle enzyme dissection of the tissue. There are technologies available such as matrix gels or extracellular matrices that function similarly to limiting dilutions. A single cell is placed in one well, and organoids are grown from this single cell.
Using this technique is also advantageous because starting with a single cell may make it easier to determine the pathogen in the cell by looking for a change in surface receptors. Overall, using a limited dilution is probably as reproducible as organoid design can be for the time being.
One audience member briefly noted that organ-on-a-chip design is generally a more easily controlled process, which means that these models are more easily reproducible and standardised. However, as they are still relatively new technology, there is not yet a systematic method for organ-on-a-chip creation.
The Viability of 3D Cell Culture Models
The discussion closed with a conversation about the viability of organoids. Currently, it is relatively easy to measure spheroid diameter, but if you want to know whether the cells are viable, more information is required. One audience member noted that they wanted to know if there was any widely used infrastructure for organoid imaging, as they had tried cutting sections from the organoids to see what happened inside them, but this technique was unsuitable as usually the spheroid will not survive.
In response to this, attendees broadly agreed that having a willingness to sacrifice organoids in order to analyse them can make the process easier, but still not “easyâ€. One reasonably successful method has been staining the outside of the organoid before using an enzyme peel to take off the outer layer and repeating this process several times.
Imaging cytometry allows far fewer cells to be used compared to flow cytometry. There are also some miniaturised machines which are similar to flow cytometry but utilise microfluid chambers to gain a deeper analysis of the cells. Overall, the most fruitful method is probably confocal microscopy.
Vandenhaute closed the discussion by touching on how the market is developing in such a way that more small fluorescent molecules are becoming available, which should make life cycle imaging easier. This is because smaller molecules such as dyes and nanobodies should be able to diffuse into the centre of spheroids, making it easier to analyse the 3D models without having to kill the spheroid in the process or use clearing techniques.
We will continue our Discussion Group series 2023 session focusing on cell line engineering, gene therapy manufacturing, next-generation strategies for gene editing, and many more. Learn more about the Oxford Global Discussion Group series at our Cell Portal.
Want to find out more about the latest stem cell therapy news? Register now for Oxford Global‘s 3D Cell Culture congress incorporates key trends and innovative technologies to accelerate the adoption of 3D models in preclinical research via advanced development, validation and application strategies.