Turbocharging Potency Assays – Challenges and Opportunities from Multispecific Drug Modalities
Presented by: Barbara Hebeis, Director of Bioassay Development at AstraZeneca
Edited by: Cara Digby-Patel
Biotherapeutics have revolutionised the therapeutic options available to patients. However, as the complexity of disease pathologies targeted increases, biotherapeutic’s complexity needs to increase, too. One means to achieve this is by addressing several disease-modulating targets within one drug molecule. The resulting multi-active or multispecific drugs provide novel challenges to chemistry manufacturing controls (CMC), particularly to the development of appropriate potency assays.
Speaking at Oxford Global’s Biologics UK: In-Person 2022 event, Barbara Hebeis, Director of Bioassay Development at AstraZeneca, discussed some of the biotherapeutic modalities that are currently in development. She offered insights into the opportunities and challenges which arise from the development of potency assays covering the complex modes of action (MoA) arising from multispecificity.
An Introduction to Potency Assays and Multispecific Drug Modalities
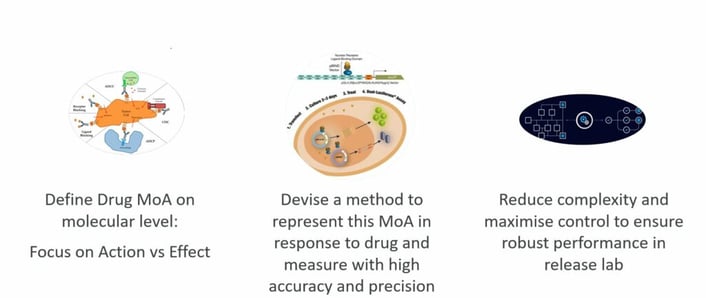
Hebeis’ primary focus fell on the early potency assay development. These stages are concerned with assessing the safety and early clinical efficacy of the drug candidate, and during this phase of the R&D process, MoA potency release and characterisation methods are developed in preparation for validation and method lifecycle management.
Potency assays for biologics have several technical requirements. Physicochemical testing is insufficient for large molecules because their clinical efficacy will depend on the molecule’s biological activity in its intended Mode of Action (MoA). The potency assays must reflect the drug MoA, which requires lot-to-lot comparison at release and either biochemical or cell-based assays depending on the phase and the MoA. The potency assays must also be simple and robust as they are often transferred to and executed by CMOs or CROs with a limited bioassay-specific scope.
A wide variety of multispecific drug modalities exists. These range from standard antibodies with bivalent binding and effector function activity, to bispecific monovalent and bivalent molecules, to far more complex molecules such as Cotadutide, a dual-active peptide which binds to two receptors across the whole molecule or viral vectors delivering several genes for protein translation to happen in the patient's tissues.
As the complexity of disease pathologies targeted increases, biotherapeutic’s complexity needs to increase, too.
There are three main MoA categories for multispecific drugs: synergy, additive, and complex. Hebeis’ primary focus was synergy, which initiates “an event which can only happen when all targets are engaged simultaneously” by targeting moiety with active moiety.
Early Clinical Phase Assays
Phases I and II of clinical development, which focus on first-time in-human (FTIH) and safety studies, can sometimes have very compressed timelines. Binding assays are usually sufficient to fulfil these stages' potency requirements: demonstration of target engagement and lot comparability, and assessment of the critical quality attribute. Early clinical studies may utilise a range of binding assays in early clinical studies (Figure 2).
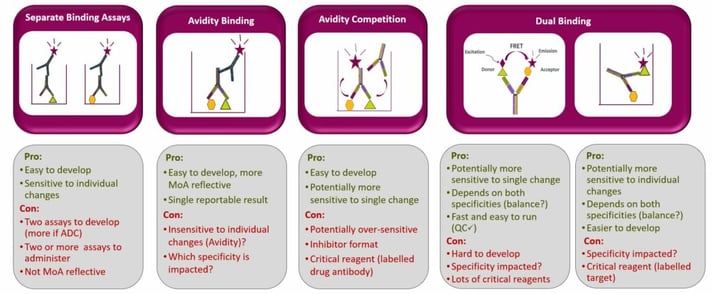
Hebeis discussed several case studies to illustrate the variety between different potency assays. For each assay discussed, the accuracy was first ensured using mock potency samples diluted to different concentrations.
In an avidity competition assay developed to reflect simple bispecific monovalent binding, it was established that the assay was sensitive to change on each individual arm; in “dummy” molecules with complete inactivity on one specificity, there was a significant reduction in potency. The assay also revealed that the method was very sensitive to degradation of the drug, for example thermal degradation also led to a loss of potency.
- Mass Spectrometry: Shortening Time Scales and Progressing Towards Automation
- Exploring & Developing Various Biotherapeutic Modalities
- Universal Vaccines & Vaccine Therapeutics
In another study, a bivalent bispecific molecule required two cell-free MoA potency assays, one for each of its specificities, due to its complexity. In absence of drug, the assay demonstrates that when substrate and either of the enzyme targets were combined, product was produced, which was measured using an analyser. When the drug was introduced and inhibited either enzyme activity by binding to them, product quantity detected was significantly decreased. This set up could then be used to measure both specificity’s potencies independently.
Despite being fully MoA-representative, this assay is less attractive for late-stage development because of its nature as two independent assays. Hebeis noted that combining the two assays into a dual inhibition assay, whilst appealing, is not currently an option because the lack of a differential substrate means there is no way to detect different products and therefore both enzyme activities in parallel.
Cell-Based MoA Assays
Phase III of the clinical development process involves the pivotal trial of the drug, demonstrating its safety and efficacy in a large patient population. Specific expectations must again be met: the relevant MoA must be fully reflected, lot-to-lot consistency must be ensured, and the manufacturing process must be established at the planned commercial site(s). A range of potency assays (usually cell-based assays) may be used for this portion of the development process (Figure 3).
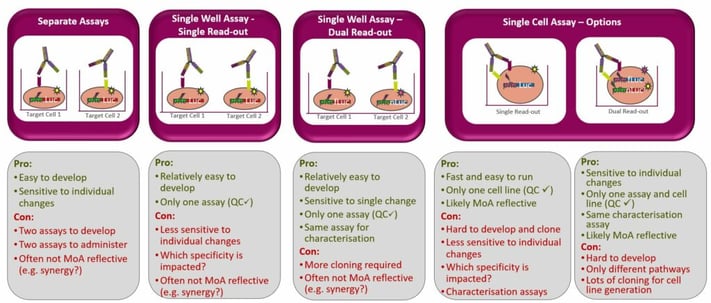
Hebeis discussed how assay development for a checkpoint inhibitor molecule might be approached. Initially, separate assays which provided single read-outs were developed. The process required a reporter cell, producing the signal, and an antigen-presenting cell line expressing all the ligands needed for the assay. This system worked well for early stages of development and demonstrated comparable potencies for the monovalent bispecific and the respective bivalent monospecific form of the antibody. However, it did not fully reflect the synergistic MoA of the drug
Therefore, researchers at AstraZeneca developed a scenario where both checkpoint inhibitor receptor sites were expressed on the same cell line. The subsequent single-cell assay, which provided a single read-out, combined the two activities of the bispecific antibody into one potency read-out. This resulted in a more MoA-representative scenario, but it suggested that the bispecific monovalent antibody was, in fact, showing a very different profile in this system than the monospecific standard antibodies combined, probably due to the bivalent binding activity they provide.
Challenges and Opportunities
Potency assay development presents several challenges. Firstly, the design, optimisation, qualification, and validation all require a thorough understanding of the drugs’ MoAs. Additionally, knowledge of their cellular and molecular mechanisms, and comprehensive exploration of the design space is needed.
Single-method potency assays may not have sufficient sensitivity to levels of decay in both target arms. In a worst-case scenario, this means that this form of assay is not feasible. Yet, even if single-method assays are viable, additional characterisation assays still need to be retained as they are crucial for showing decay pathways for each moiety.
Opportunities presented by the process include the possibility of developing a single potency assay for release and stability testing of a multispecific molecule. Hebeis noted that maintaining and executing a single assay would be the most “QC-friendly” approach. In most instances, this also results in more MoA-reflective biology. Ultimately, Hebeis expressed hope that new technologies for measuring pathway activation will be better suited to combination and differentiation than traditional reporter assays, as this streamlines the assay development process.
Join Oxford Global’s annual Biologics UK: In-Person event today. This 3-day conference brings together a panel of prominent leaders and scientists, sharing new case studies, innovative data, and exciting industry outlooks.