T Cell Engagement and Activation: New Developments
In the ever-evolving landscape of cancer therapeutics, innovative approaches that harness the body's immune system to target tumours have gained significant attention. Among these groundbreaking strategies, cell engagers have emerged as a promising frontier, offering new hope in the fight against cancer. These agents leverage the natural cytotoxic abilities of immune cells to selectively eliminate malignant cells, regardless of the tumour type or its inherent immune evasion mechanisms.
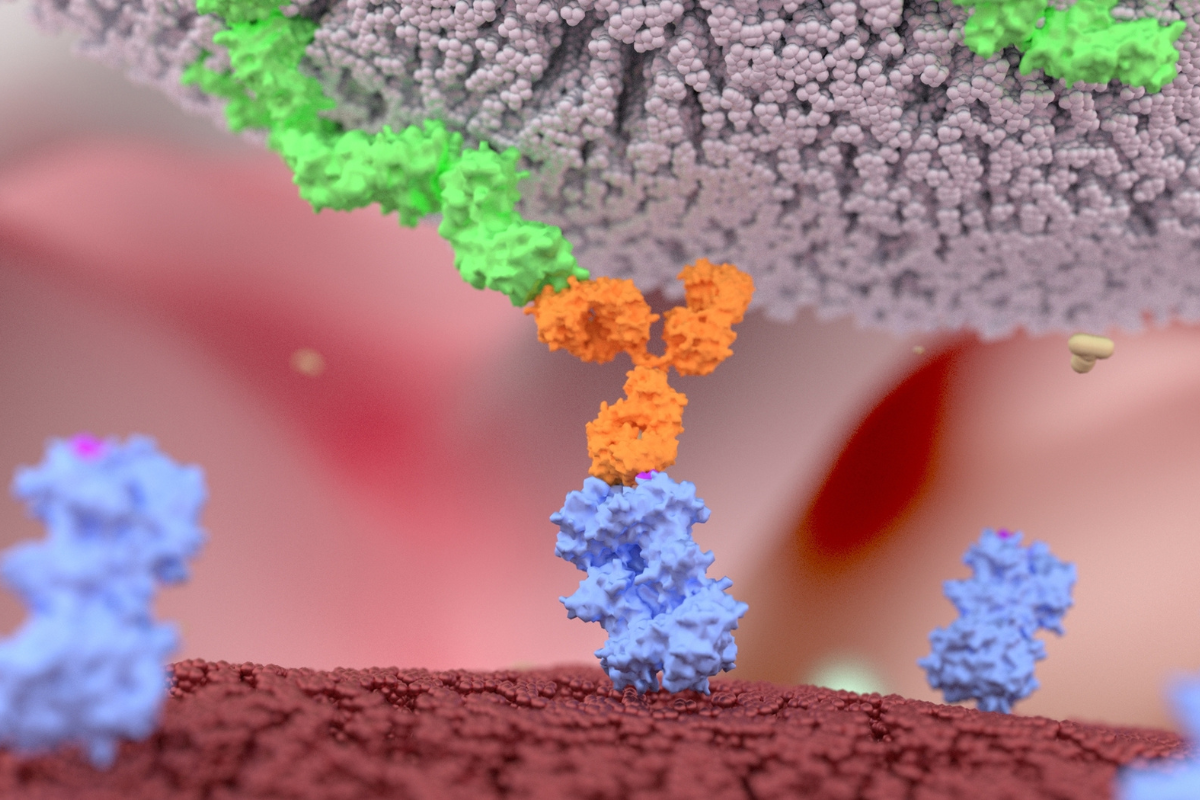
This article delves into the transformative potential of cell engagers, specifically focusing on the extraordinary capabilities of bispecific antibodies. By bridging immune cells and cancer cells, bispecific cell engagers facilitate a direct connection, enabling the immune system to mount a potent attack against tumours. This dual-targeting approach enhances the specificity and efficacy of immune cell activation and opens new avenues for therapeutic interventions in various cancers, including hematologic malignancies and solid tumours.
In presentations at Oxford Global’s Biologics 2023 and Immuno 2023 events, we saw cutting-edge research on new T cell engager technologies presented by industry experts. In this Insight Article, we look at two of these presentations, delivered by Dan Snell, Vice President of Translational Sciences at Numab Therapeutics, and Rob Roovers, Senior Director of Preclinical Development at LAVA Therapeutics.
Presentation 1: T Cell Engager Design and Combinations
Presented by: Dan Snell, Vice President of Translational Sciences at Numab Therapeutics at Biologics 2023
Numab Therapeutics, a clinical-stage multispecific antibody development company, is revolutionising cancer therapeutics through its proprietary pipeline of next-generation antibody-based therapeutics. The molecules designed by Numab have improved stability thanks to an engineering technique they have developed, whereby they alter the sequence of the light chain of their single chain variable fragments. This step occurs alongside the humanisation process and allows the molecules to be reliably used as the basis for multispecifics therapeutics.
Snell discussed two of Numab’s lead molecules which demonstrate this improved stability and safety profile. He began by presenting on NM28, a mesothelin-targeting T cell engager. Mesothelin expression is associated with various difficult-to-treat cancers, including mesothelioma and pancreatic cancer.
NM28-2746 is a bivalent molecule that can discriminate between tumour cells expressing high levels of mesothelin and normal cells, which typically express low levels of the protein. As Snell put it, Numab has been working to “create a window of opportunity for the treatment, to be able to target cells with high densities of mesothelin.” Cell viability assays and T cell activation assays demonstrated that NM28 exhibited increased potency on tumour cells whilst sparing normal cells, thanks to its bivalent binding and higher complex stability.
Numab Therapeutics has also developed NM32, a TCE targeting ROR1, a protein associated with solid tumours and hematologic malignancies. NM32-2668, a monovalent ROR1 molecule, ensures constant efficacy irrespective of target expression levels. It induces specific killing of non-proliferating chronic lymphocytic leukaemia (CLL) patient samples and demonstrates tumour regression in a mantle cell lymphoma model.
- The Bispecific Antibody Landscape
- Computational Tools and AI/ML for Antibody Engineering
- Bispecific CAR-T Cell Therapies: Successful Applications in Mouse Tumour Models
For both molecules, researchers at Numab explored their potential as combination immunotherapies. Strong anti-tumour activity was observed by combining NM28-2746 with a PD-L1/4-1BB bispecific antibody. This combination ultimately resulted in the remodelling of the tumour microenvironment, as it led to an increase in the number of CD3+ T cells and a decrease in CD4+ T cell counts within the tumour, thereby creating a hostile tumour microenvironment.
Similarly, when NM32 was combined with the same bispecific, researchers again observed strong anti-tumour inhibition, reflecting a high efficacy of engaging ROR1 at low dosing when in combination with PD-L1/4-1BB.
This research shows the potential of combination therapies using T cell engagers. Numab’s groundbreaking approaches hold great promise in addressing the challenges posed by diverse tumour types and advancing towards more effective and personalised cancer therapies.
Presentation 2: Development of V?9V?2 T Cell-Engaging Bispecific Antibodies for Efficacious Cancer Treatment
Presented by: Rob Roovers, Senior Director Preclinical Development at LAVA Therapeutics at Immuno 2023
V?9V?2-T cells, a subset of ??-T cells, have emerged as critical players in antitumor immunity. These cells exhibit consistent pro-inflammatory cytotoxic effector functions and unique antigen-presenting abilities. Studies have shown that the presence of V?9V?2-T cells in tumour tissue is associated with a favourable prognosis for cancer patients.
LAVA Therapeutics is a biopharmaceutical company that has leveraged the potent antitumor capabilities of V?9V?2-T cells to develop a bispecific CD123 targeting V?9V?2 T cell engager. CD123, also known as interleukin-3 receptor-alpha, is expressed in many haematological malignancies, including acute myeloid leukaemia and Hodgkin's lymphoma.
Roovers explained how the bispecific V?9V?2 T cell engager aims to enhance T cell engagers' specificity and therapeutic window. Unlike traditional approaches, this novel engager aims to retain potency while increasing tumour specificity and avoids the activation of regulatory T cells. Additionally, it can potentially stimulate downstream immune activation such as antigen presentation.
LAVA has employed two formats for their bispecific V?9V?2 T cell engagers. The first format utilises a bispecific single-domain antibody, which exhibits high affinity binding and potency. This molecule, smaller than regular IgG1 and BiTE formats, is being used in their haematological program. The second format combines a bispecific single-domain antibody with an Fc domain. This format also demonstrates high affinity binding and potency, with an in vivo half-life similar to canonical IgG1. It is being employed in their lead solid tumour program.
In preclinical studies, the V?9V?2 T cell engager has shown promising results. The engager binds to CD123 and exhibits high potency in killing CD123+ tumour cells. Importantly, it demonstrates tumour cell selectivity, relatively sparing CD123+ healthy cells from lysis. This selective targeting indicates that the therapy has the potential to be studied clinically.
Furthermore, the CD123 targeting V?9V?2 T cell engager developed by LAVA does not block IL-3-induced proliferation, indicating that it may not interfere with the normal function of IL-3, which is a crucial driver of haematopoiesis.
Overall, LAVA's bispecific CD123 targeting V?9V?2 T cell engager reflects a “strong scientific and clinical rationale for tumour targeted engagement of V?9V?2 T cells,” thanks to the potential for an improved safety profile of such therapies. LAVA has already finished its cell line development and begun preclinical development. They expect to be ready to file a clinical trial application for the molecule in 2024.
Want to know more about Oxford Global’s upcoming Vaccines Europe event? Download the agenda or register today to join prominent leaders and scientists as they share new case studies, innovative data, and exciting industry outlooks.