mRNA Therapeutics: Learning from Preclinical Concepts to Ensure Clinical Translatability in Nanomedicinal Approaches

Presented by: Michael Keller, Expert Scientist at Roche
Edited by: Ben Norris
Messenger RNA (mRNA) has recently emerged as a promising class of nucleic acid therapy, with the potential to induce protein production to treat and prevent a range of diseases. Ionisable lipid nanoparticles (LNPs) are used to encapsulate mRNA for delivery, but their application in vitro may not fully replicate the barriers they encounter in vivo. By designing and evaluating a library of engineered LNPs, healthcare providers and coordinators of mRNA therapeutics can deploy successful treatments such as the Covid-19 vaccine.
Dimensional Differences in mRNA Vaccines
As Michael Keller, Expert Scientist at Roche explained to the audience at Oxford Global’s Biologics UK: In-Person event in March 2022, mRNA therapeutics require delivery. “Thanks to the current pandemic, some of the really successful vaccines use this technology to deliver this really long fragment of RNA,” said Keller. “This could be something 4,000, 5,000 nucleotides in length, so not anything we are used to working with in the small molecules space.”
- Industry News: Moderna Sues Pfizer and BioNTech over mRNA COVID-19 Vaccines
- The Black Box Effect – How Can AI and ML Provide Transparent Insights for Drug Discovery?
- Widening the Therapeutic Window: Developing Antibody Drug Conjugates for Solid Tumours
Due to the dimensional differences inherent to mRNA vaccines, they require a delivery system. LNPs provide that delivery system. Vaccines based on mRNA-containing LNPs are a promising new platform for future treatments, used by two leading vaccines against Covid-19. LNPs function as carrier vehicles to protect the chosen mRNA from degradation and to aid both intracellular delivery and endosomal escape.
Antisense Oligonucleotides in mRNA Synthesis and Delivery
Keller explained that this project started with an ongoing programme using transition electron microscopy, which found visible trends in the structure of the lipids and sRNA nanoparticles. "We can clearly see differences in the architecture of these particles," said Keller. He highlighted the presence of a molecular arrangement. “This protects the LNP from serum and other extracellular components, and also keeps them together,” said Keller.
Antisense oligonucleotides (ASOs) are another class of oligonucleotides that have been successfully used to regulate mRNA for therapeutic purpose (outlined in Figure 1). In contrast to mRNAs, ASOs are short, synthetic oligonucleotides that can alter RNA and modify protein expression. Since they are chemically very different to mRNAs, the learnings made from the LNPs that deliver the mRNAs may not necessarily apply to ASOs. “One of the most important questions is how we select the best LNP,” continued Keller, “as well as the rationale behind that choice.”
Double or Nothing: Dual Formulations in Parallel
As Keller highlighted, a major drawback with this avenue of mRNA therapeutics is the possibility of the programme falling at the last hurdle. A failure to meet the endpoint in a clinical trial results in a high probability of the programme being terminated. “Do we have the appropriate pre-clinical data?”, asked Keller. “These are the kinds of questions we have to consider.”
Keller explained that the translation of preclinical research into clinical readouts with oligonucleotide based therapies is not straightforward. “We decided it’s worth to pursue several formulations in parallel and let the data speak at the final readout,” he said. “Obviously it’s more front-loaded, with more cost at the beginning,” said Keller, “but when you count in from a cost and capacity and time perspective from when you have to terminate the programme then it works out as more cost effective.”
Another focus of the workaround was the selection process for ionizable lipids, using a barcode library as a selection tool. Keller explained that an ionizable lipid library was generated based on successful literature reports, and that this technology allows screening hundreds of LNP formulations concomitantly to identify relative leads that are most successful for a given application.
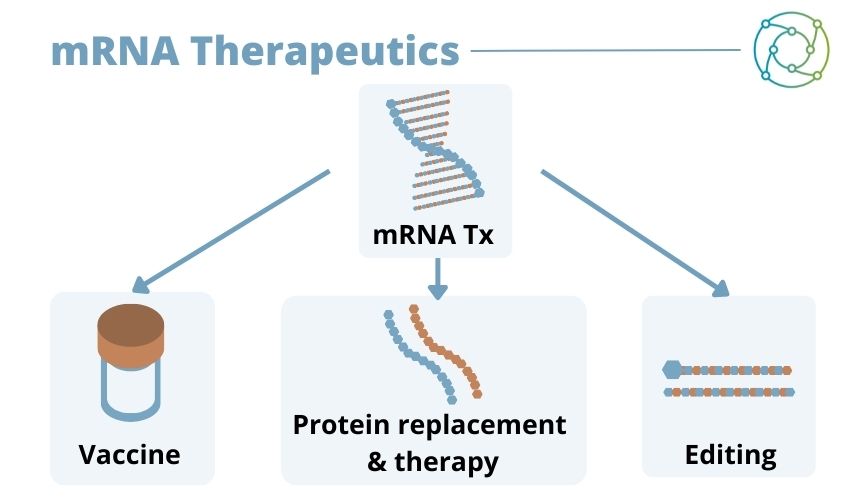
“You can use a very elegant system where you can formulate each of these ionisable lipids into individual entities,” said Keller, “then you put them together in one mix using a barcoded nucleic acid system for investigation.” From here, a desired therapeutic response can be obtained, be it for vaccines, protein replacement therapies, or gene editing techniques.
mRNA Therapeutics and Areas of Application
There are upsides and downsides to working with mRNA vaccines, as with all avenues of biologics research. One of the main advantages is the fast generic discovery rate innate to the process, which helps to provide rapid development along with the existence of established formulation principles for vaccines. However, delivery is a current drawback: at present there is no successful LNP with active targeting, and biodistribution is limited, with uncertain applicability for mRNA editing.
Rounding off, Keller attributed the commercial presence of microfluidics technologies as making process development much easier, revisiting the barcode technology previously explored to select leads from a large pool of LNPs. “There is a high complexity from concept to product,” said Keller. “We want to minimize surprises when it comes to final readouts in terms of efficacy and toxicity.”
Want to learn more about new developments in healthcare and therapeutics? Head over to our Biologics portal for up-to-date insights from the industry’s best and brightest. If you’d like to register your interest in our upcoming Oligonucleotide Chemistry & Therapeutics Symposium, visit our event website.







