Insulin: Recombinant Production, Sustainability, and Chemistry
Biologics 2023 included a pre-event day which featured panels and presentations focusing on sustainable chemistry for proteins and peptides. One such presentation was by Thomas Høeg-Jensen, Scientific Director at Novo Nordisk, and explained how his company had implemented recombinant peptide production techniques with chemical and enzymatic transformations in order to reduce its carbon footprint.
Høeg-Jensen outlined the difference in toxic chemical waste and CO2 emission between using a recombinant process and a solid-phase peptide synthesis (SPPS) process. As an organic chemist, Høeg-Jensen said that he recognises the value in SPPS due to its broad range of applications, such as the many protecting groups, schemes, ranges of solvents, and reagents. “You can really design the molecule to your need,” he said. However, the SPPS process comes at a cost of very high CO2 and waste production.
On the other hand, recombinant production uses microorganisms in water; the waste from these fermentations can also be used to produce animal feed and fertiliser. Its CO2 emissions per year are also far lower. Therefore, recombinant production of peptides is much more environmentally sustainable.
The Need for Insulin Production
Høeg-Jensen works with insulin, its production, and synthesising new types thereof for improved applications. Insulin is a peptide with 51 amino acids, 2 chains, and 3 disulphides. The drug recently celebrated the 100-year anniversary of its discovery in 1921, before which a diagnosis of diabetes was considered a death sentence. For many years, the peptide was extracted from animal pancreases until human insulin was able to be synthesised in a lab.
RELATED:
- Peptide Production: Biopharmaceutical Manufacturing of Next Generation Peptides
- The Future of Peptide Chemistry and Sustainability
A long-acting crystalline form of insulin was developed in the 1940s but was only active in the body for 12 hours after a dose. Diabetes patients need insulin active in their body 24 hours a day, ideally with a spike every time they eat. This means that the drug can be difficult to dose, with type I diabetes patients needing to dose themselves with every meal, adjusting the dose depending on what they eat.
Furthermore, getting the dose wrong can be disastrous in some cases. Too much insulin can lead to a hypoglycaemic state which can be deadly; too little leads to high blood sugar levels, which comes with long term risks like blindness and cardiovascular damage. This means that diabetes patients have to learn to be their own doctors and adjust their doses every day.
The first chemical transformation of insulin turned pig insulin into human insulin using an enzyme; either achromobactor lyticus protease (ALP) or trypsin. “Although medically, pig insulin works fine in humans, it was a major turning point when humans could have human insulin available,” said Høeg-Jensen. When the peptide is made recombinantly, the same enzyme is used to turn a recombinant precursor into human insulin.
Novo Nordisk uses yeast to produce this recombinant precursor. Yeast is particularly advantageous because it can continuously excrete folded proteins. This is opposed to E. coli-based production, which is a batch process with an additional refolding stage.
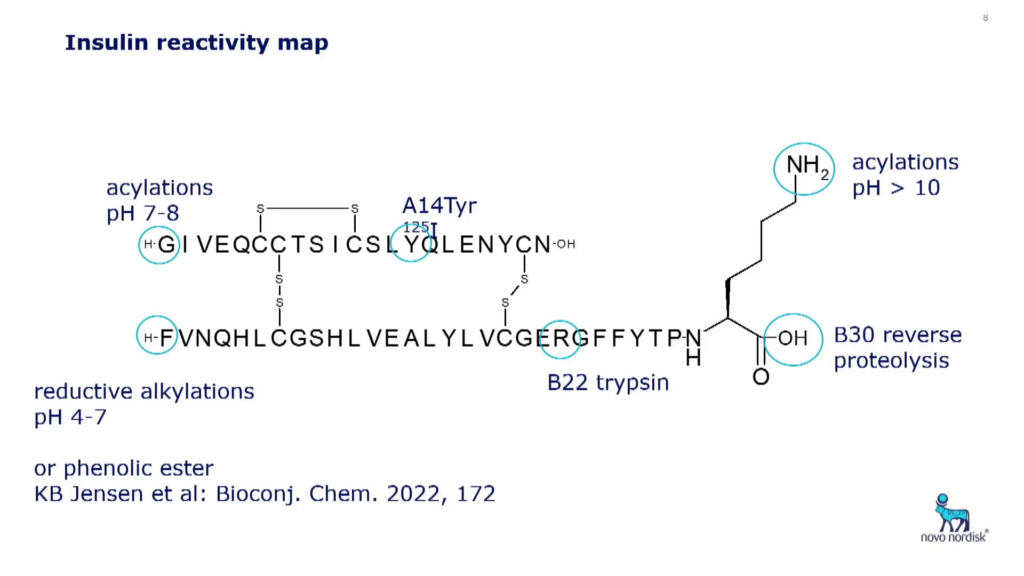
Insulin’s Structure, Enzymatic, and Chemical Modifications
The molecular structure of insulin has only one lysine, and two end termini with different pKa values. This means that if a chemical reagent is used at pH greater than the pKa value of the ? side chain, for example chemical reactions with an active ester, the main product will be at the B29 side chain. “Scaling this process up to a factory setting can produce a very high yield,” said Høeg-Jensen. The first chemically modified insulin, detemir, uses this process and includes a fatty acid at B29.
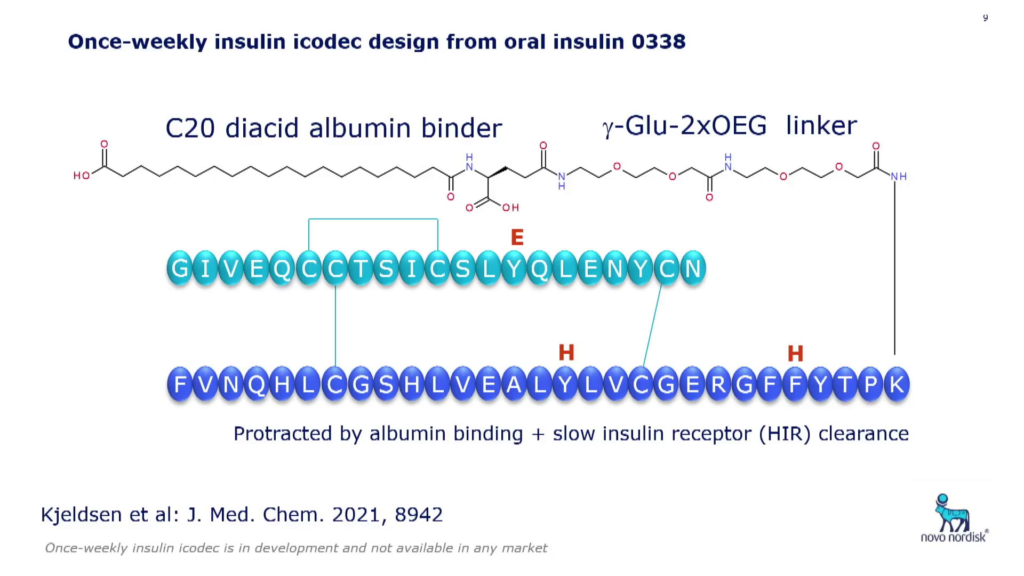
Example: Insulin Icodec
One of Novo Nordisk’s latest emerging insulin products is a once-weekly candidate that has just completed Phase III clinical trials and is now under registration. Insulin icodec is an insulin form with a C20 fatty diacid albumin binder and short PEG-like linker + ?-Glu linker (see Fig. 2).
Three mutations to icodec’s backbone were originally added to increase its enzymatic stability, but it was also discovered that this lowers the receptor affinity which contributes to the prolonged action of the drug. Compared to regular insulin, its receptor affinity is 30,000-times lower, giving it a 160-hour half-life. Høeg-Jensen said that this product was mainly intended for type II patients, due to the difference in insulin excretion patterns of the two types of diabetes patients.
Example: Oral Insulin
Another example that Høeg-Jensen gave was an attempt by Novo Nordisk to create an oral form of insulin. The product made it to clinical trials and worked well. However, the bioavailability of the product was found to only be around 5% — with 95% of the drug going to waste in the body. Due to the fact that the price of insulin is roughly around 1,000€ per patient per year of treatment, the company ultimately decided that it was too wasteful to allow 95% of the product to be lost.
RELATED:
Therefore, increasing the oral bioavailability of insulin has been on Novo Nordisk’s radar. Cyclosporin is an oral cyclic peptide drug which prompted chemists to wonder whether insulin could be made into a cyclic peptide. Initial results showed that they were able to make the necessary modifications using ALP to produce this cyclic insulin. Although it did not make sense in the end to develop the concept further, it was still a successful proof-of-concept.
Conclusions
Høeg-Jensen concluded that even though recombinant production of peptides can be limited in chemical diversity, there are a wealth of chemical and enzymatic steps available to add new sidechains or chemical groups to insulin precursors. The fatty acid acylation that was pioneered by Novo Nordisk’s detemir has become a primary platform for protein and peptide prolongation in the industry. Furthermore, Høeg-Jensen regards enzymatic transformations as fantastic tools; ALP being the “crown jewel.”
The field still has more to learn and there is presently a need for better methods for conjugations to tryptophan (Trp) or methionine (Met) or for expression systems with non-natural amino acids. “The latter technology is currently not mature enough to do this at production scale, only really for model compounds,” Høeg-Jensen explained.
This article was written by Tom Cohen.
Original presentation and review by Thomas Høeg-Jensen.
Join Antibody Engineering 2023: Online – An intensive 2-day meeting that delves into the latest in antibody engineering and antibody-based therapeutics & a meeting place for experts working within engineering, computational tools and antibody-based therapeutics.






