In Vivo Model Design and Assay Selection to Aid in Progression from Preclinical to Clinical

Dr Matthieu Daugan is Associate Director of Cell-Mediated Immunity at Nexelis, a Q2 Solutions Company. Before Nexelis, Matthieu worked for PAIRimmune, transferring to Nexelis’ preclinical department in 2021, and then to the cell-mediated immunity clinical team. Therefore, Daugan has experience leading teams in both clinical and preclinical fields.
In Nexelis’ presentation at Vaccines EU 2022, Matthieu described vaccine development as “a complex road to success.” Once a clinical candidate has been identified, there are many steps that preclinical and clinical teams need to go through to translate that lead into a successful product. This is where services from CROs come in, helping with lead optimisation, preclinical, clinical, and operational testing. “Each of these steps implicates the generation of large datasets, leading to key go/no-go decisions,” said Matthieu.
- Nexelis, a Q2 Solutions Company, Measuring Immunogenicity and Vaccine Efficacy
- Nexelis’s Collaborative Development for Optimal Clinical Assay
- Vaccines After COVID: How the Pandemic Has Changed the Vaccination Market
“Let’s assume that you finally have in your hands a promising candidate and you are really looking forward to entering the preclinical phase,” Matthieu suggested. The preclinical stage is the next critical phase after discovery. During this stage, multiple preclinical setup questions need to be asked, all of these parameters will have immediate and long-term impact when it comes to the downstream clinical setup.
“Which clinical model should we use? Which clinical endpoint should be chosen? Which assay should we select and how should they be developed? How can I ensure a smooth transition from the preclinical application to the clinical application?” Matthieu explained that making the right choices will either facilitate or complexify the remaining path of development.
What Preclinical Decisions Make a Difference in the Clinical Stage?
Matthieu demonstrated this idea by talking us through the case of an influenza vaccine’s preclinical development. There is a plethora of models to choose from to study the influenza virus — all with their own strengths and weaknesses (Matthieu took us through some of these options, see figure 1).
| Model | Advantages | Disadvantages |
|---|---|---|
| Mouse | - Many immunological tools are available. - Less variability due to same genetic background. - Transgenic models are available. Cost. | - Not naturally susceptible to the infection. - Adaptation of the virus is required in most cases. - Pathogenesis is different than in humans. - Infection mostly in the lower respiratory tract. - Frequency of lymphocyte in mouse and humans are different. |
| Guinea Pig | - Naturally susceptible to human strains. - Cost. | - Do not show a lot of symptoms. - Limited availability of immunological reagents. |
| Hamster | - Naturally susceptible to human strains. - Cost. | - Do not show a lot of symptoms. - Limited availability of immunological reagents. |
| Ferret | - Naturally susceptible to human strains. - Many similarities with human for the anatomy, physiology of the respiratory tract. | - Limited availability of immunological reagents. - Cost. |
| Pigs | - Many similarities with human for the anatomy, physiology, and genome. - Immunological reagents are becoming more available. - Symptoms resemble those observed in humans. | - Cost. - Special requirements for housing and manipulation. - Now partially overcome with minipig model. |
| Non-Human Primates | - Many similarities with human for the anatomy, physiology, and immunological response. - Naturally susceptible to human strains. - Immunological reagents are becoming more available. - Symptoms resemble those observed in humans. | - Ethical consideration. - Cost. - Special requirements for housing and manipulation. |
The vast multiplicity of tools available at the preclinical stage introduces the stand-out question of which model to use at the preclinical stage of a project. “The path toward the generation of a vaccine is complex and can be divided into multiple steps,” said Matthieu. “More specifically, the preclinical stage can be divided into two steps: proof-of-concept, and the true pre-clinical stage.”
The goal of the proof-of-concept stage is to identify the potential candidate, understand how the immunity is triggered, characterise the candidate, and characterise the immune response. As the proof-of-concept stage is very early on in the process, cost considerations are very important here.
The true preclinical phase should characterise the vaccine’s efficacy to induce an immune response and to protect the animal against a pathogen. Further tested here are the candidate’s dose regimen and safety.
Choosing Animal Models
Choosing an appropriate animal model will be influenced by the stage of the study and the question that the researchers need to answer. Matthieu outlined the criteria that he thought makes a successful animal model (figure 2). He stressed that although there are specific criteria for a perfect animal model, there is no perfect model and there will always be differences. “No animal model will meet all these criteria.”
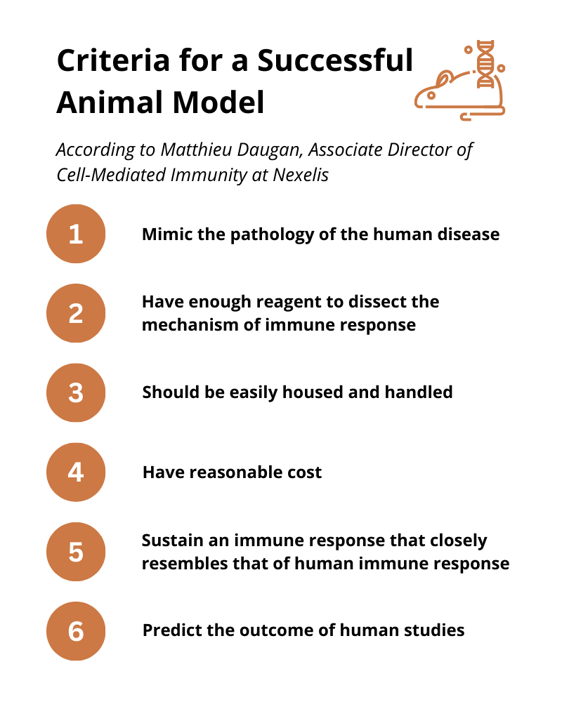
Proof-of-Concept Stage
At the proof-of-concept stage, mouse models are often used. This is because mouse reagents are very easily accessible, well characterised, and predictable. The use of inbred mice will decrease the heterogenicity of the response and will allow the team to draw clearer conclusions. Furthermore, mouse models are easy to procure in groups large enough to have the statistical power to draw conclusions at a low cost. Matthieu said that these models will not necessarily provide a direct prediction of what will happen in humans, which is not necessarily the objective at this stage.
True Preclinical Stage
The true preclinical stage should ideally provide a prediction of the candidate’s effect in humans. To choose the right model for the job, this is when the team needs to consider the factors associated with each animal model and the question being asked by the model.
“For example, does the question ask whether the vaccine protects against the transmission of the virus? Then the mouse and ferret models would be more preferable than the monkey model, in which the transmission is very low,” explained Matthieu.
“Or is the question whether the vaccine protects against the development of the disease? Then the ferret model would be better than the guinea pig model, in which the animals do not develop a lot of symptoms,” he added.
Challenges and Lessons Learned
“Animal models are very various, each with their own pros and cons,” said Matthieu. Making the choice as to which animal model to use will depend on the stage of the study, the intrinsic characteristics of each model, and the question that is being asked by the study and that will be answered later on in the clinical stage.
As a result, Matthieu said that it was “paramount to consider the finality of the candidate to test before you select a model.” The goal of the clinical phase of development will ultimately drive the choice of the in vitro assays that will complement the in vivo part.
Collaborative Assay Development Process for Clinical Applications
The next part of the presentation focussed on the assay development process for clinical applications, referring to the case study of the SARS-CoV-2 vaccine development. Nexelis has used an inter-departmental approach in order to accelerate the process of assay development. Their preclinical, clinical, and protein sciences teams have all worked in collaboration to reduce dependence on external providers and easily transition from the preclinical to the clinical phase.
Matthieu explained that the critical materials were generated in-house by their protein science team. This meant that Nexelis were able to achieve a faster turnaround, independent of external providers, and offered the opportunity to create pilot-batch production to assess the yield and purity of those materials.
Next was the immunisation phase from the preclinical department which generated the early development panels and controls. “Once again, this allowed us to have early access to controls, standards, and sample panel material,” Matthieu explained. This allowed Nexelis a greater degree of control on both the quality and quantity of material available to develop their assays.
“After that, instead of being handled by the clinical department from A to Z, the assay development was started by the preclinical department so that it could be applied to both departments,” Matthieu continued. To ensure the alignment between preclinical and clinical teams on the requirement for downstream communication, this required upstream communication.
Nexelis favoured functional assays such as pseudotyped virus neutralisation assays due to their non-specificity toward species, which made for ease of translation to clinical. “We also used other types of assays,” said Matthieu. “But we really pushed for the non-specific assays.”
Those assays were then transferred to the clinical team, using the preclinical reagents that were generated by the preclinical team. Matthieu noted that “this allows for an easy adaptation of preclinical assays for human use and the assay performance was already defined in the preclinical stage.” This gives Nexelis enough time for human reagents to be available for clinical testing and qualification downstream.
Once the qualification and validation steps are done, the clinical operational team helps smoothen the transition by participating in the final steps. Further collaboration was done by the protein sciences team by providing characterised lots of key reagents (e.g., antigens and viral-like particles (VLPs)). Throughout the assay’s entire life cycle, its performance was also monitored.
Join Antibody Engineering 2023: Online - An intensive 2-day meeting that delves into the latest in antibody engineering and antibody-based therapeutics & a meeting place for experts working within engineering, computational tools and antibody-based therapeutics.
This article was edited by Tom Cohen.
[elementor-template id="29448"]






