Gilead Pay Big Bucks for MacroGenics' Blood-Cancer Targeting Bispecific

On Monday October 17th, Gilead and MacroGenics announced an exclusive collaboration agreement for the development of the bispecific MGD024 and two additional bispecific research programs. The deal stipulates that Gilead will dish out $60 million upfront for the rights to later licence the bispecific, whilst MacroGenics will be eligible to receive up to $1.7 billion in target nominations, option fees, and development, regulatory and commercial milestones..
- Eisai’s Alzheimer’s Drug Offers New Hope
- The STING in Immunotherapy’s Tail
- Is Your WASH Causing Inflammation? Protein Complex Linked to Inflammatory Conditions
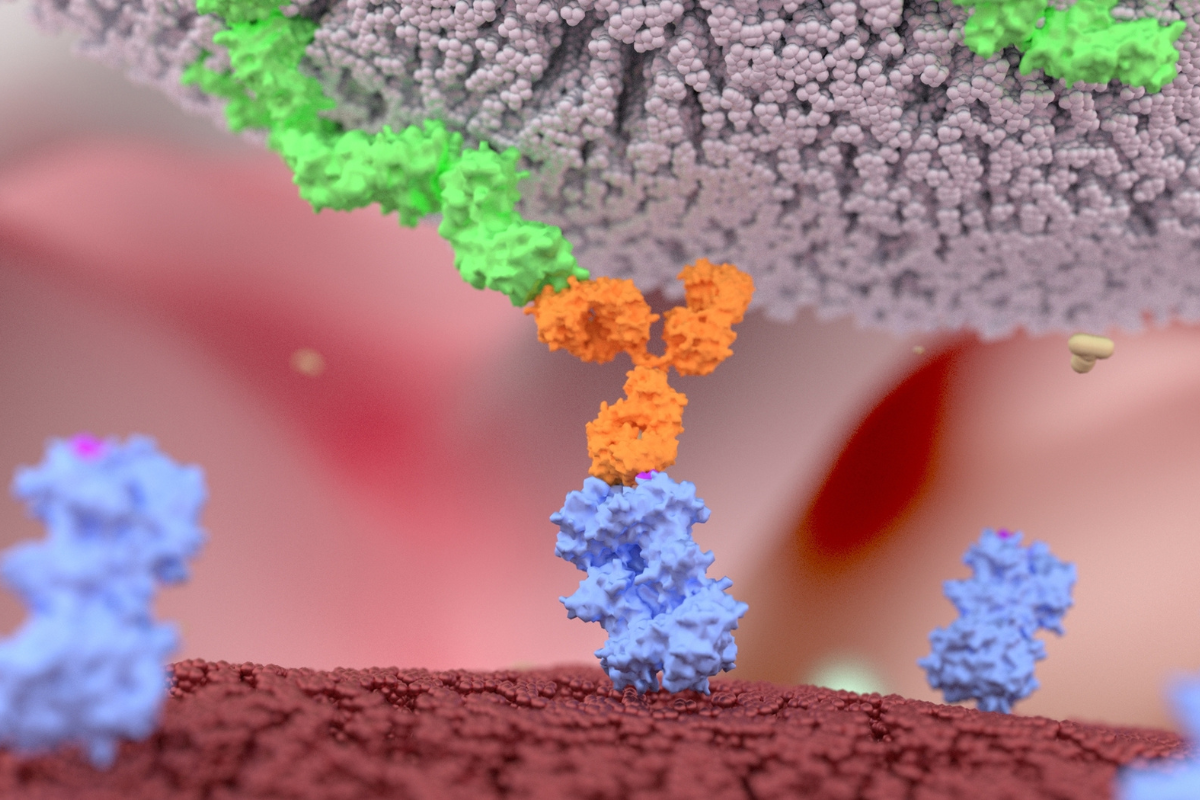
MGD024 is an investigational bispecific that binds to CD123 and CD3 with the aim of invigorating the immune system to wipe out CD123-expressing cancer cells. In the official Gilead press release, MGD024 was described as a “next-generation bispecific” which is purported to specifically minimise cytokine-release syndrome (CRS), an inflammatory condition characterised by an overreaction from the immune system.
Moreover, whilst increasing anti-tumour activity, MGD024 also has a longer half-life, which will allow for intermittent dosing. Bill Grossman, Senior Vice President of Oncology Clinical Development at Gilead, described this trait as having the capacity to result in "more patient-friendly dosing and enhanced clinical outcomes” for people living with haematological cancers such as myeloid leukaemia (AML) and myelodysplastic syndromes (MDS).
In February, MacroGenics decided to shift their focus from flotetuzumab onto MGD024 development using their DART platform, which produces molecules with more stable structures. Their ongoing Phase I study will include a dose escalation segment, and an expansion segment, designed to evaluate the bispecific both as a monotherapy and in combination with other therapies across several indicators. During this Phase I stage, Gilead may exercise its option to licence the program at predetermined decision points.
MGD024 was described as "a next-generation bispecific" which is purported to specifically minimise cytokine-release syndrome
CD123 has been widely recognised as “a very promising target in oncology research”, as Scott Koenig, President and CEO of MacroGenics, noted. Indeed, multiple drugmakers have pursued CD123 because of its potential in haematological cancer treatment, including Affimed and ImmunoGen.
However, because CD123 sits on both healthy and cancerous cells, developing drugs to target the receptor is not without its challenges, as the treatment window is relatively narrow. This isn’t necessarily a bad thing for MacroGenics and Gilead, though; other firms’ failings mean that the market is still relatively open, which bodes well for MGD024 if it finds clinical success.
Join Oxford Global’s annual Biologics UK: In-Person event today. This 3-day conference brings together a panel of prominent leaders and scientists, sharing new case studies, innovative data, and exciting industry outlooks.
Get your weekly dose of industry news here and keep up to date with the latest ‘Industry Spotlight’ posts. For other Biologics content, please visit the Biologics Content Portal.