Biologics US 2024

3 - 4 October 2024 | San Diego, CA
Driving the discovery & development of safe, effective & innovative biologic drugs through streamlined discovery, clinical and analytical strategies

Explore Biologics US 2024: Overcoming the Complexities of Biologics Development
Biologics have come to the forefront of the pharmaceutical industry, with ever-increasing demand for large molecule products, but their development still presents unique and complex challenges from both technical and regulatory perspectives. Join 550+ leaders from across the US biologics industry at Biologics US to discuss the innovative strategies & technologies needed to enable more effective biological products to enter the market.
Across two days, it features novel case studies from all corners of the biologics industry, with dedicated programmes covering next-generation Protein & Antibody Therapeutics, Peptides, and Antibody-Drug Conjugates. Alongside innovative presentations, our curated programme allows for in-depth networking – fostering new partnerships that will advance your pipeline and help you translate your innovative target from bench to bedside.
Proteins & Antibodies US Congress 2024
From new engineering technologies and AI/ML-guided approaches through to novel analytical development methods and success stories in NextGen therapeutics, the Proteins & Antibodies US Congress provides an all-encompassing look at the back-bone of the biologics industry.
Agenda at a Glance
Day One | Track 1: Antibody Discovery & Engineering
Day One | Track 2: Bioanalytical Development
Day Two | Track 1: Computational Tools, AI/ML-Guided Engineering
Day Two | Track 2: NextGen Therapeutics – Multispecifics, Cell Engagers, & Other New Modalities
Peptides US Congress 2024
Peptide drug development has made huge progress in the last decade, with advancements in production, modification and analytical technologies. With this rise in investment and innovation continuing to increase, our dedicated Peptides Congress showcases novel data in peptide CMC, delivery and drug development, supporting you as you look to optimize complex molecules.
Agenda at a Glance
Day One | Track 3: Peptide Discovery & Development
Day Two | Track 3: Peptide Formulation, Delivery & Manufacturing
ADC Discovery & Development US Congress
2023 proved to be a monumental year for the ADCs industry, with high-profile collaborations between biopharma organizations and innovative new molecules reaching the clinic. The ADC Discovery & Development Congress seeks to advance ADC development further by maximizing efficiency and minimizing toxicity through safe, effective and streamline preclinical, clinical and CMC strategies. Further sessions look at the next-generation of conjugates, showcasing new approaches that could tackle different disease areas where there is currently unmet need.
Agenda at a Glance
Day One | Track 4: Engineering Conjugates to Enhance Therapeutic Potential
Day One | Track 5: Advancing Analytical Methods
Day Two | Track 4: Translational & Clinical Lessons
Day Two | Track 5: Manufacturing, CMC & Process Development





Networking & knowledge-sharing is at the heart of what we do. Alongside the innovative programme, attendees can engage in a variety of event features and make the most of participation in a range of experiences
-
Over 120 dynamic interactive discussions, roundtables, and presentations curated with insights from top industry leaders and experts
-
Global Technological Showcase
An international exhibition showcasing cutting-edge technologies and services from leading providers in the scientific field. -
Networking Emphasis
Unparalleled networking opportunities, including speed networking sessions, a vibrant drinks reception, and informal gatherings to foster meaningful connections -
Innovative Poster Displays
Inspiring poster displays and presentations unveiling the latest breakthroughs from emerging biotech, pharma, and academic institutions
Companies Represented Include












Meet Our Expert Speakers
Biologics US 2024 again brings together a panel of prominent leaders and scientists, sharing new case studies, innovative, data and industry outlooks.
Below are some of our featured speakers:




All Speakers




































Meet Our Esteemed Sponsors
Gold Sponsors

Silver Sponsors
Bronze Sponsors
Network & Programme Sponsors
Interested in Sponsoring?
Oxford Global’s range of sponsorship opportunities are highly effective in enabling market immersion and visibility, with direct access to clients and potential customers.
Learn moreLatest Resources
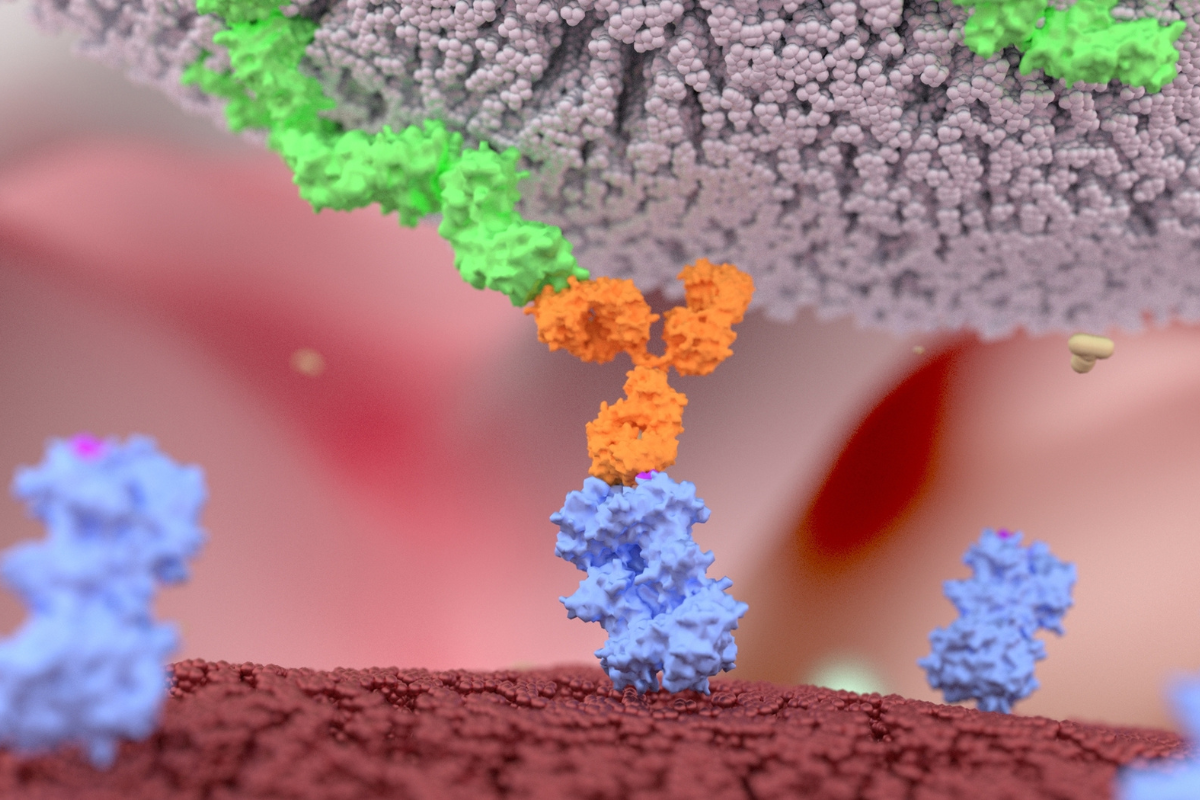
Merus's Preclinical Bispecific Zeno Demonstrates Efficacy in Cancer Models
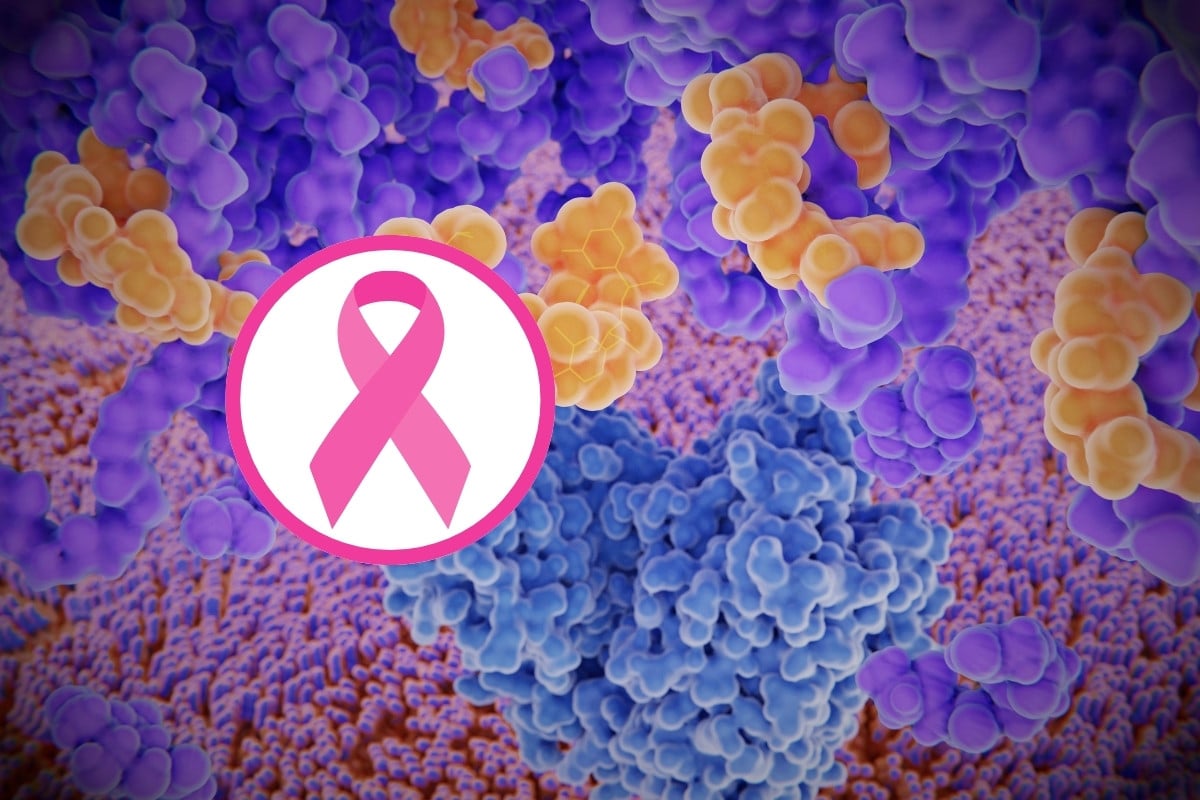
New peptide with hope of treating triple-negative breast cancer
Overcoming the Hurdles: Navigating the Challenges of Bioconjugate Development

The Current Landscape of ADC Approvals and Market Trends: Q&A With Mahendra Deonarain, CEO of Antikor Biopharma

Sustainable Pharma: Celebrating Progress and Confronting Challenges on Earth Day

Rapid Analytical Approach for Biologics Characterization Using Mass Photometry
We are closely monitoring the official guidance from health authorities, local governments, and the World Health Organization in order to support the health and well-being of our global community. The health and safety of our staff, customers and clients remains our number one priority.
As we continue to move forward with hosting our events in-person in 2024, we’ve added a series of Health & Safety guidelines and precautions in order to prepare for event safety. We carry out risk assessments for all our events to evaluate fundamental considerations and how to cover multiple risk scenarios.
Oxford Global has learned that third-party companies (recently EHotel Services, Business Travel Management/btravelmanagement and Exhibitors Hotel Reservations Services) are targeting conference attendees with a fraudulent hotel booking scheme.
Please note that none of these third-party companies are associated with Oxford Global in any way, nor have Oxford Global authorised them to use their names or trademarks on information they send out to attendees.
If you are contacted by a third-party company by phone or email using Oxford Global’s name or the name of Biologics US 2024 and offering accommodation services, we urge exhibitors and attendees to proceed with extreme caution before signing anything sent by these companies or entering into any conversation or replying to any emails sent from these third-party companies.
Register Your Interest
Submit your details and a member of our team will be in touch





.png?length=600&name=73739cc8-e311-4fbb-a7dd-68ee123f5d12-2-5668375logo-1.GenScriptCorporation_Logo_EN_Horizontal_FullColor-(2).png)



