mRNA Manufacturing: Scalability and Process Analytical Techniques
The clinical application of mRNA remained out of reach for nearly two decades due to issues with instability, immunogenicity-related toxicity concerns and scalability. Another issue that prevented the widespread adoption of mRNA technology was the need for a cold storage delivery chain. Fortunately, chemical modification, innovative carriers and new manufacturing methods are overcoming these issues.
Delivery: Lipid Nanoparticles
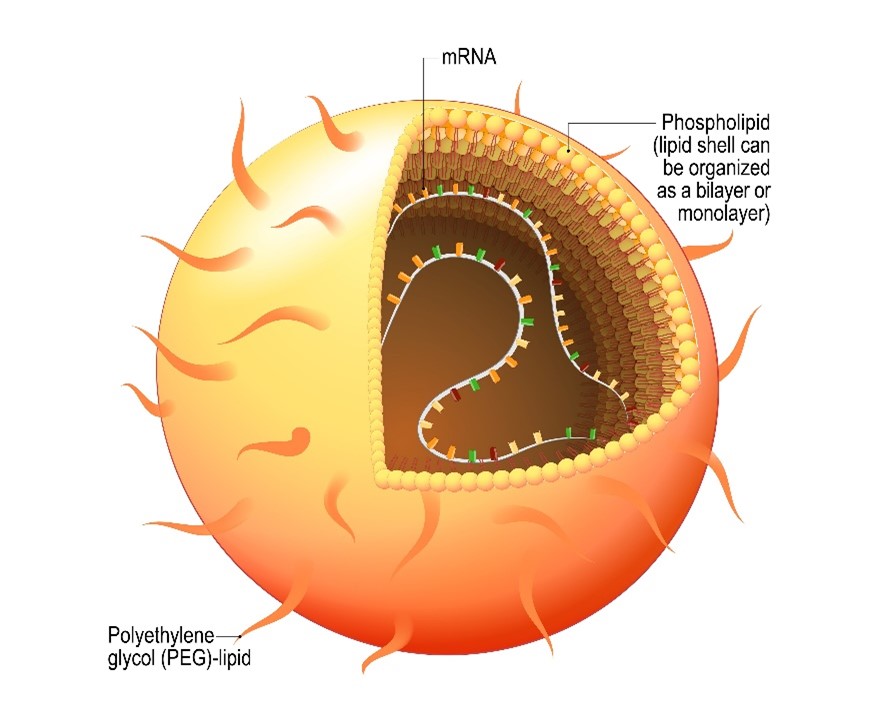
Various materials have been developed for mRNA delivery, including lipids, lipid-like materials, polymers, and protein derivatives. Lipid nanoparticles have been thoroughly investigated and successfully entered the clinic for the mRNA. Notably, two COVID-19 vaccines, mRNA-1273 and BNT162b21, use lipid nanoparticles to deliver antigen mRNA. In addition, many other lipid nanoparticle mRNA formulations have been developed and are under clinical evaluation to prevent and treat virus infections, cancer, and genetic diseases.
To be successful, lipid nanoparticle carriers for mRNA need to overcome many obstacles. Challenges include nuclease degradation, interception, clearance, and the accuracy of to target issue and cells. Finally, mRNA molecules must escape endosomes to reach the cytoplasm, where translation occurs. Until recently, the demand for lipid nanoparticles was low, and there were few facilities and established supply chains.
Fortunately, most excipients for nanoparticles are well understood and are relatively simple to produce. However, in some cases (for example, the Pfizer/BioNTech and Moderna mRNA vaccines) production requires require crucial excipients that are much more difficult to make. Therefore, these companies need specialised manufacturing expertise, equipment, and facilities to create under good manufacturing practice (GMP) conditions at the high-quality level necessary.
Manufacturing Advances
In the past, lipid nanoparticle formulations were produced by thin-film hydration and reverse-phase evaporation. Extrusion techniques were then applied to limit variability in size.
Today, Lipid nanoparticle–mRNA formulations are mainly produced using rapid mixing techniques. This involves mixing lipid components and mRNA molecules using tightly controlled flow rate and pH levels. Crucially, this allows lipid nanoparticle–mRNA formulations to be reproduced with high encapsulation efficiency and homogeneous size distribution, improving scalability. Furthermore, due to the COVID-19 pandemic, many CMOs (contract manufacturing organizations) rapidly expanded their lipid nanoparticle manufacturing capacity.
mRNA Manufacturing: Purification & Scale Up
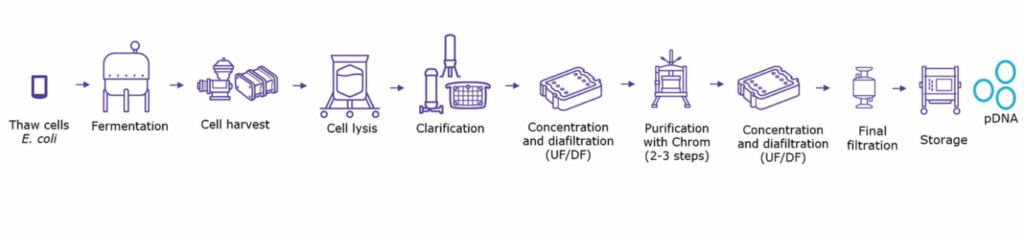
mRNA manufacturing is a complex process split into many stages. The creation of plasmid DNA templates is a crucial first step. Based on these DNA templates, the mRNA is then transcribed in vitro in the presence of an RNA polymerase and ribonucleoside triphosphates. The residual DNA template is removed by DNase digestion, and chemical or enzymatic methods cap the mRNA molecules. Unfortunately, these DNA templates are easily contaminated with E. coli, RNA, proteins, and endotoxins, necessitating purification steps through the manufacturing process.
Process Control Analytics
Purification and formulation consist of a series of unit operations, each with its own operating requirements and performance parameters. Establishing a benchmark or expected result for each unit operation and real-time measurement of the unit’s performance can reduce the frequency of failed batches. In addition, critical process failures can be traced back to the exact unit operation and batch of material where a deviation occurred. Product stability, purity and quality can be assessed using several analytical techniques, such as gel electrophoresis and high-performance liquid chromatography (HPLC).
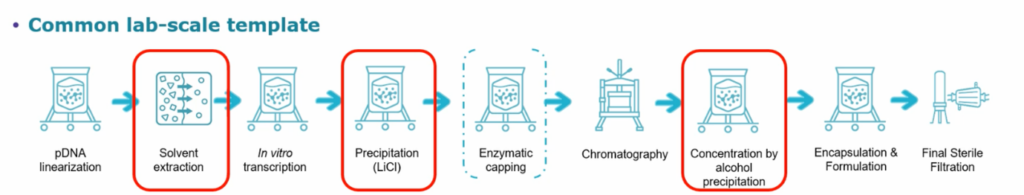
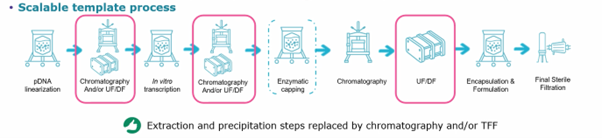
Chromatography & Scalability
David Gemmel, MSAT Biomanufacturing Engineer at Merck, explains that “solvent extraction or precipitation steps are problematic at lab scale. They utilise solvents, which is not ideal once you scale up to commercial volumes and operate within a GMP environment. They also have significant scalability issues. The solvent extraction and precipitation steps can be swapped with chromatography or tangential potential flow filtration (TFF). These technologies scale incredibly well, so once you’ve done your proof-of-concept step using solvent extraction and other techniques, you should start utilising devices for chromatography and TFF because it provides much better predictability at larger volumes.”
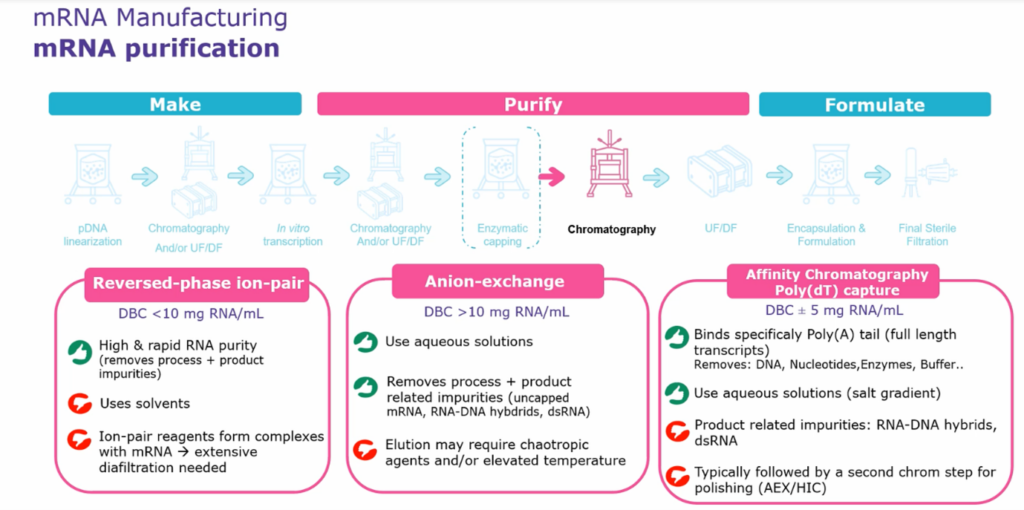
mRNA Purification
One crucial step in mRNA manufacturing is RNA purification. A common first step is to use DNase digestion that separates DNA strands into much smaller fragments, which can then be purified using TFF, with cut-offs of between 30 and 200 kilodaltons. This is required because of the risk of DNA fragments hybridising with mRNA, causing a significant impurity that must be removed.
At this stage, the aim is to remove DNA fragments, endotoxins, wholesale proteins and restriction enzymes before moving downstream. Chromatography is typically used as a final purification step. There are three main options, each with benefits and downsides. It is vital to understand your impurity profiles and select a strategy that minimises time and financial requirements. Chaotropic agents are toxic and can cause harmful side effects so their removal must be thoroughly validated.
Reversed-Phase Ion-Pair
Reverse Phase Ion-Pair provides an excellent level of impurity removal quickly. However, it does use solvents. At GMP scale this is problematic, and potentially pair reagents may form complexes with the mRNA. This ultimately means that extensive diafiltrating is needed, increasing your process cycle time and the amount of buffer you need. In turn, this can lead to much higher costs.
Anion Exchange
Anion exchange utilises aqueous solutions, which is much easier to work with than solvents. It can remove process and product impurities, such as uncapped mRNA, DNA hybrids, and double-strand RNA. When using anion exchange, you may require chaotropic agents and elevated temperatures. Unfortunately, chaotropic agents can cause changes to the properties of the proteins in your solution, while elevated temperatures carry the risk of degrading mRNA and other delicate biologics.
Affinity Chromatography Poly(dT) capture
The final option is using affinity chromatography step with a poly DT capture resin that will bind specifically to your poly (A) tail. This will remove any DNA, nucleotides, enzymes, and buffer impurities. It uses aqueous solutions, but it will not renew or remove product-related impurities. The main problem this causes is that you will need a second chromatography step to remove RNA and DNA hybrids and double-strand RNA.
Final Thoughts & Conclusion
mRNA took a long time to come to fruition, but recent advancements has enabled mRNA manufacturing at a never seen before scale. Now, more than ever, researchers and pharmaceutical companies have a multitude of options to trial and test. The success of mRNA COVID-19 vaccines has finally bought mRNA into the limelight and opened new doors to future vaccines and therapeutics.
If mRNA manufacturing and delivery are of interest to you, take a look at our events page for more information on ‘RNA Therapeutics and Delivery US: In-Person.’ This two-day event will bring together key opinion leaders to share their research and cutting-edge presentations on the latest innovations in RNA formulation & clinical development. For more information, visit the event page here.