Migraine Drug Doubles its Sales, Despite Expansion Setback

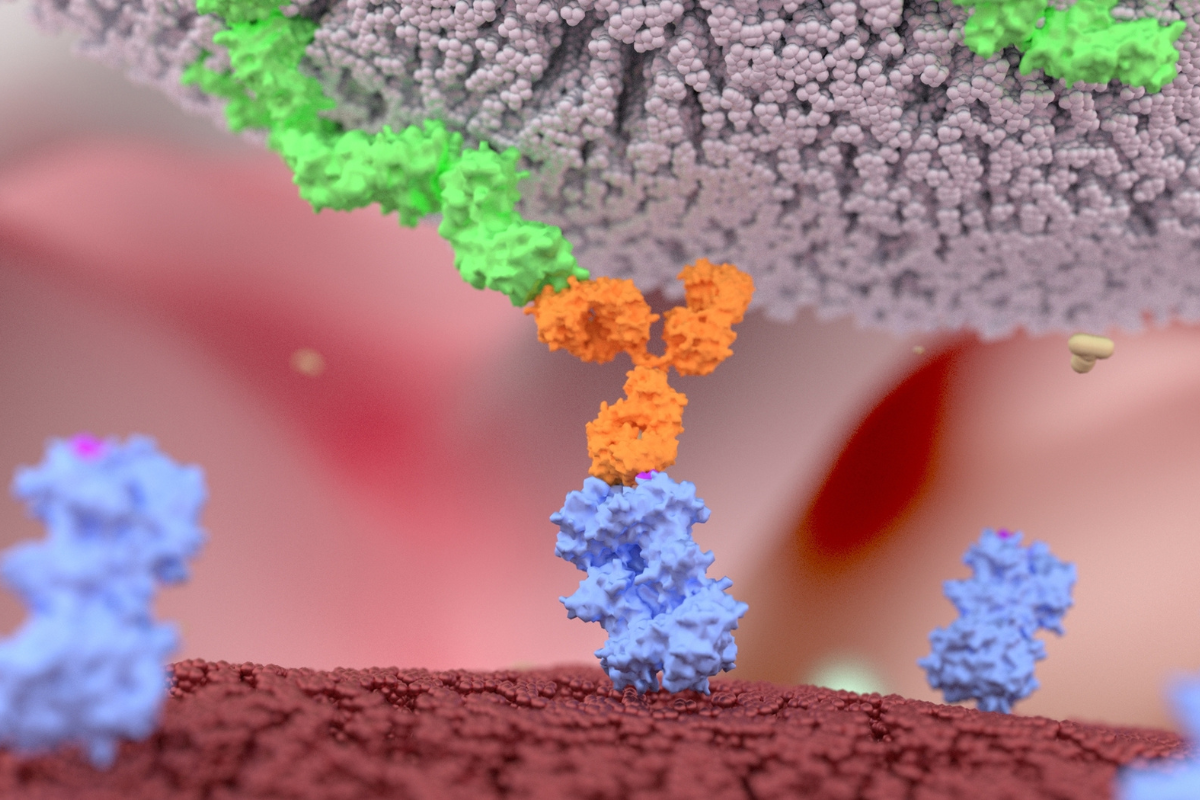
Lundbeck’s calcitonin-gene-related peptide (CGRP) medication, Vypeti, has achieved record sales despite suffering setbacks in its expansion plans. Vypeti is a monoclonal antibody that targets and blocks the binding site of the CGRP – the protein thought to cause migraine pain.
Vypeti is administered via an IV infusion, whilst other approved CGRP migraine treatments on the market are administered as jabs. These include Aimovig, Ajovy, and Emgality. NURTEC ODT is the latest CGRP medication to achieve approval and takes of form of an oral pill.
Vypeti received FDA approval in 2020 for the preventative treatment of migraines in adults. It has not been established whether the drug is safe and effective for children or patients over 65.
- Will Vaccine Manufacturers go into Overdrive this Flu Season?
- Peptide Discovery: Unlocking the Future of Disruptive Therapeutics
- Achieving Oral Delivery of Peptides
Lundbeck received the go-ahead from the European Commission in January of 2022 and has since rolled out the drug in Australia, Singapore, and Switzerland. It aims to achieve eight additional market rollouts by the end of this year.
However, the promising CGRP inhibitor hit a snag in its market expansion plans when it failed to significantly outperform a placebo in reducing the number of days per month patients suffered from migraine headaches. The phase III trial called Sunlight was conducted predominately in China and tested patients with a combined diagnosis of chronic migraines and medication overuse headaches.
On Wednesday last week, Johan Luthman, Head of R&D at Lundbeck, announced that the migraine prevention trial was designed as an “accelerated path” to launch in China. Now, the Danish company has been forced to return to its base strategy to once again try and achieve regulatory approval across China.
Lundbeck received the go-ahead from the European Commission in January of 2022 and has since rolled out the drug in Australia, Singapore, and Switzerland.
Luthman described the strategy as being “a very aggressive trial for exploring what is possible for China.” Despite recent disappointments, it looks like Vypeti has weathered the storm. In fact, the drug’s profit sales appear to have gone from strength to strength.
From 2020 to the first half of 2022, Vyepti’s sales have jumped 120 % to reach over 390 million Danish Krone (DKK). So far, 220 million DDK has been reported for the second quarter of this year.
Join Oxford Global’s annual Biologics UK: In-Person event today. This 3-day conference brings together a panel of prominent leaders and scientists, sharing new case studies, innovative data, and exciting industry outlooks.
Get your bi-weekly dose of industry news here and keep up to date with the latest ‘Industry Spotlight’ posts. For other Biologics content, please visit the Biologics Content Portal.