Key Insights: Scaling Up Pharmaceutical Lyophilization Processes

Our April 2022 Formulation Series discussion group focused on lyophilisation and the challenges for scale-up. Oxford Global’s discussion groups bring together a select group of 15-20 key industry leaders for approximately one hour for in-depth knowledge sharing and conversation.
This month the group was led by Claudia Krichbaum, Principal Scientist, Pharmaceutical Technologies, at Merck Healthcare KGaA. Krichbaum has worked with Merck since 2017. She is responsible for drug product formulation and process development for injectables, specialising in lyophilisation.
Krichbaum opens the group by laying out one of the key aims for improving lyophilisation process development in the pharmaceutical industry, explaining that “the goal is to scale up the freeze-drying cycle from laboratory to pilot scale, which will be used in general for clinical supply and later scale up to manufacturing scale, which will be used commercially.”
It is crucial to understand that the freeze-drying cycle should be optimised for the equipment used at the manufacturing scale, not the laboratory. This includes considering the specific limitations of the freeze dryer in general as well as evaluation of differences between the freeze-driers at different scale.

Identifying Addressing Scale-Up Issues
Development and optimization of the freeze-drying cycle in the pharmaceutical industry is vitally important to cut costs for consumers. But, unfortunately, much like mRNA manufacturing, high volumes present new obstacles.
Due to the sensitive nature of lyophilisation, it’s very important to characterise and account for the different resources and equipment you will use during scale up to the commercial phase. Krichbaum advocates for advanced experimental characterisation of the equipment at each scale to know specific limitations. While this brings increased knowledge about facilities, the disadvantages are that the experiments may be time consuming, cost intensive, and that data interpretation requires experienced experts. Nevertheless, at the manufacturing scale, mistakes due to a lack of knowledge can be far more expensive than characterisation costs.
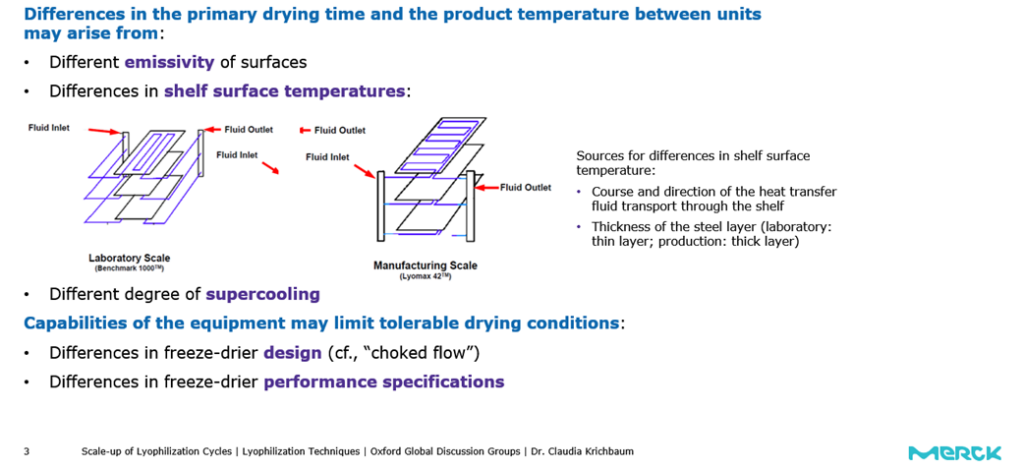
The literature available shows that primary drying times are generally longer and result in higher product temperatures at manufacturing scale units. There are various reasons which could cause these variations.
Krichbaum gives some examples, including “differences in surface emissivity and shelf surface temperatures. Also, heat transfer via radiation may be higher in the laboratory. If your freeze dyer has an acrylic door, this is even more important. At manufacturing scale, you are likely to use stainless steel which lowers rates of heat transfer.”
The degree of super cooling is also important. It is generally higher at the manufacturing scale. Krichbaum explains that “this means the product nucleate at lower temperatures, resulting in very small ice crystals and these ice crystals leave smaller pores. So, the escape of water vapour via sublimation of water from your product is slower at manufacturing scale.”
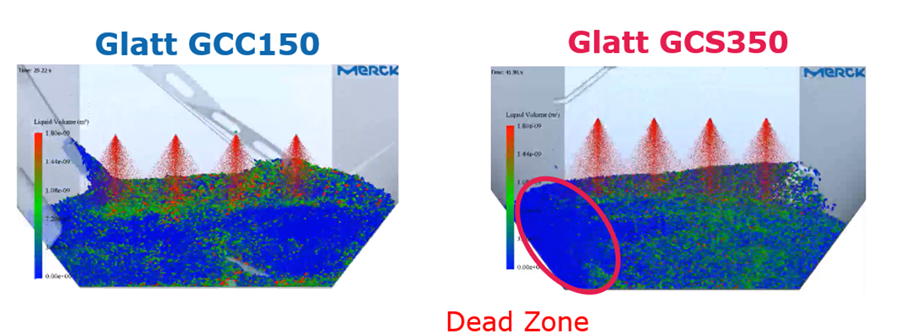
Choked Flow & Sublimation tests
One of the worst consequences that can occur when equipment is not properly characterised is “choked flow”. This is when the equipment cannot handle the mass flow during the primary drying phase, possibly resulting in a loss of pressure control. As the pressure decreases, the water flow rate speeds up and can continue until the velocity of water vapour reaches the speed of sound. Potential conseqeunces include decrease in sublimation rate and increase in product temperature. This might ultimately lead to collapse of the product cake and loss of the batch.
Sublimation tests with water can be used to predict and prevent the occurrence of choked flow. Krichbaum explains that “the maximum transfer rate is a function of freeze-drier design and condenser capacity, and these numbers can help predict the combination of shelf temperature and chamber pressure which might result in choked flow conditions. These kinds of experiments provide increased knowledge about your freeze dryers; however, they are really time-consuming. You also need specialist knowledge or an experienced expert to know what to do with the results from these experiments.”
Final Thoughts & Conclusions
At Oxford Global, we could not be more pleased with the turnout and feedback from this discussion. This meeting provided the perfect setting for exchanging ideas, sharing innovations, and discussing the ever-evolving digital therapeutics landscape. If you would like to join one of our future groups, please take a look at our upcoming events page. For more on lyophilisation, join our dedicated full day lyophilisation symposium coming this November.
References:
- 1.Guidance for Industry, PAT—A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), U.S. Government Printing Office: Washington DC, September 2004.
- 2.Tang X, Pikal MJ. 2004. Design of freeze-drying processes for pharmaceuticals: practical advice. Pharm. Res. 21(2):191-200.
- 3.Tsinontides SC, Rajniak P, Pham D, Hunke HW, Placek J, Reynolds SD. 2004. Freeze drying-principles and practice for successful scale-up to manufacturing. Int J Pharm 280 (1-2):1-16.
- 4.Tchessalov S, Warne N. 2008. Lyophilization: cycle robustness and process tolerances, transfer and scale up; European Pharmaceutical Review, (3): 76-83.
- 5.Schneid SC, Gieseler H. 2011. Rational approaches and transfer strategies for the scale-up of freeze-drying cycles. Chemistry Today, Vol 29 (1): 43-46.
- 6.Rambhatla S, Pikal MJ. 2003. Heat and mass transfer scale-up issues during freeze-drying I: atypical radiation and the edge vial effect. AAPS Pharm Sci Tech: 4 (2), 111-120.
- 7.Rhambhatla S, Ramot R, Bhugra C, Pikal MJ. 2004. Heat and mass transfer scale-up issues during freeze drying: II. control and characterization of the degree of supercooling. J Pharm SciTech 5(4):1-9.
- 8.Rambhatla S, Tchessalov T, Pikal MJ. 2006. Heat and mass transfer scale-up issues during freeze-drying, III: control and characterization of dryer differences via operational qualification tests. AAPS Pharm Sci Tech 7, E39.
- 9.Pikal MJ. Quality by Design and scale-up issues in freeze drying: the role of controlled ice nucleation. SP Scientific LyoLearn Webinars, www.spscientific.comLyoTech-CenterLyoLern-Webinars-Archive.
- 10.Patel SM, Chaudhuri S, Pikal MJ. 2010. Choked flow and importance of Mach I in freeze-drying process design. Chemical Engineering Science 65, 5716-5727.
- 11.Patel SM, et al. 2017. Lyophilized Drug Product Cake Appearance: What Is Acceptable? J Pharm Sci 106(7):1706-1721
- 12.Pikal MJ, Roy ML, Shah S. 1984. Mass and heat transfer in vial freeze-drying of pharmaceuticals: role of the vial. J Pharm Sci 73(9):1224-1237.
- 13.Hibler S, Gieseler H. 2010. Primary packaging materials for pharmaceutical freeze-drying: molded vs. serum tubing vials. Eur Pharm Rev 15(4):10-16.
- 14.Hibler S, Wagner C, Gieseler H. 2012. Vial freeze-drying, part 1: new insights into heat transfer characteristics of tubing and molded vials. J Pharm Sci 101(3):1189-1201.