Embracing The Power of RNA Therapeutics

RNA therapeutics have experienced tremendous interest in the past few years, and COVID-19 has increased this trend. RNA therapies that utilize mRNA are being used to develop personalized cancer vaccines and vaccines for infectious diseases such as the Zika virus. Researchers are also exploring whether these types of treatments can be used as protein-replacement therapies and monoclonal antibody expression.
History of RNA Therapeutics
At Oxford Global’s RNA Vaccines workshop in 2021, Tod Woolf gave an insightful presentation on the early days of the field and how it has developed. Tod Woolf’s research teams have focused on biologics & RNA Therapeutics. His teams co-developed the MOD mRNA Therapeutics™ and Vaccine platform, STEALTH RNAi™, Self-Delivering RNAi™ and Therapeutic Editing™.
He founded Sequitur in 1996, which Invitrogen acquired in 2003.? He currently serves as CEO of ETAGEN and is the Executive Director of Technology Ventures at Beth Israel Deaconess Medical Centre in Boston.
Early Development
Woolf explained that “our work started in 1996-1997 started but was long proceeded by Malone, Falconer, and Burma. In 1989, they were researching plasmid DNA for gene therapy and also did experiments with mRNA. But they didn’t focus on chemically modified mRNA.”
Woolf recounted the reaction to the initial proposal to use mRNA, during his time at Sequiur. “The idea was to put the RNA in a liposome, deliver it, and then it would translate and make proteins. This may seem obvious now. In reality, even in 1996, when we spoke to people about this, they looked at us like we were crazy. They asked why you would want to use mRNA? It’s so unstable; why not just use DNA?”
Advantages of RNA compared to DNA
RNA has some advantages as either a therapeutic or vaccine, or even a research tool, compared to DNA-based transfections or gene therapy. First, it’s gene free, so there is no integration and no long-term effects.
Woolf explains that “from a safety standpoint, that’s an advantage. Another is that there are no immunogenic vector proteins. It’s pretty much as pure as you can get if you want to express a protein in the cell to get a T cell response. You want it to be expressed inside the cell, not just a protein, but something that’s expressed in the cell and will be presented on the surface for T cell responses.”
mRNA Delivery of Monoclonal Antibodies
Also speaking at Oxford Global’s RNA Vaccines workshop, Wade Blair shared his team’s progress on using mRNA to induce monoclonal antibody expression. Wade Blair is Head of Virology and Vaccine Discovery Vaccines and Immune Therapy R&D BioPharmaceuticals at AstraZeneca
Blair contributed to the discovery and/or development of several approved drugs and molecules in late-stage clinical trials, including the HIV protease inhibitor atazanavir (Reyataz); the HIV non-nucleoside reverse transcriptase inhibitor doravirine (Pifeltro); the HIV attachment inhibitor fostemsavir (Rukobia); the HIV capsid inhibitor lenacapavir; and letermovir (Prevymis), a treatment for cytomegalovirus (CMV) infections.
Rationale and Motivation
Blair explains that his team look at their work as a complementary addition to vaccines. He explains that “our goal here was to devise or generate an integrated platform to deliver recalling pandemic prevention treatments, in a very short time. One might imagine during an outbreak that the antibody or the antibody delivery could provide immediate protection to patients while they’re waiting for the immune system to kick in. Another application is in the immune-compromised, or folks that don’t respond well to vaccination; this can provide protection to those populations as well.”
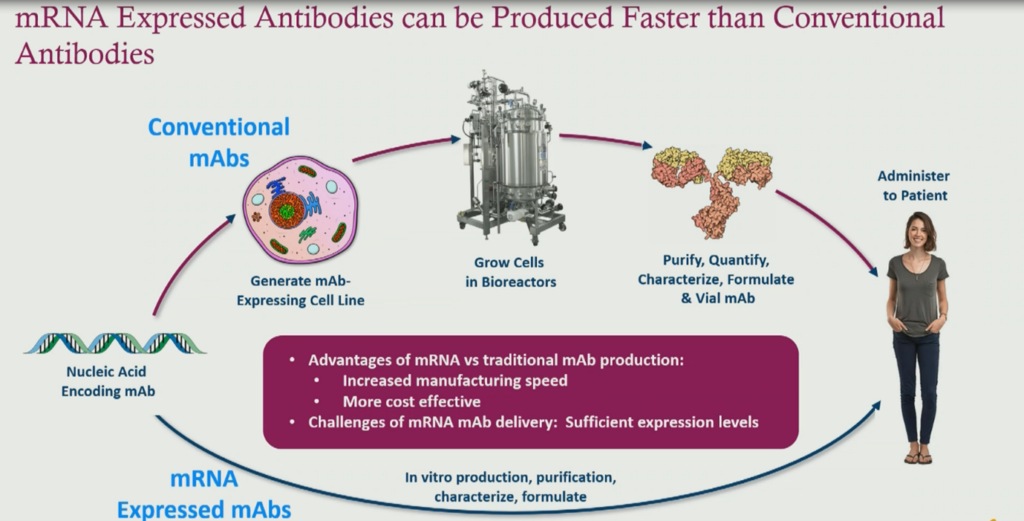
Advantages Compared to Traditional Monoclonal Antibody Development
The traditional production of monoclonal antibodies is relatively slow. Blair explains that “developing an antibody requires developing a cell line amplifier growing a cell line or scaling it up in bioreactors, purifying the antibody and then delivering it. This actually takes quite a bit of time, where mRNA is much faster. The majority the process in vitro is formulated synthetically and can be delivered much more quickly than then a traditional antimony approach. And again, this is something that is a huge advantage when responding to a pandemic outbreak. There’s also the potential cost of goods advantages with mRNA.”
Final Thoughts & Conclusion
Despite initial scepticism, RNA therapeutics have proven to be effective for a wide range of diseases and conditions. We are still in a pivotal era of the field, with many new and exciting research projects underway to discover novel applications. For more on RNA, consider joining us for RNA Therapeutics & Delivery US: In-Person or RNA Therapeutics & Delivery Europe: In-Person.