Digital Biomarkers: Addressing Unmet Needs

The spread of wearable digital technologies in healthcare created a new type of medical information: digital biomarkers. Digital biomarkers are quantifiable, physiological, and behavioural data collected and measured through consumer digital devices. Examples include sensors, wearables, implantables, and mobile phones. The goal is to use this data to create actionable insights into the biological state of individuals.
Unmet Needs: The Rationale for Digital Biomarkers
It now takes approximately 14 years from target identification to an approved drug reaching the market, a timespan that has increased in the past few years. Trial duration for phase two and phase three studies are particularly long, while sample sizes are also increasing. For a drug to be approved, it needs time and large pools of patients to show if a treatment has been effective.
The lack of measurement techniques that can prove efficacy is one reason for the slow pace. Digital biomarkers provide the ability to measure participants much more frequently. Traditional clinic visits cannot observe fluctuations in patient disease costs, which can lead to mischaracterisation of symptom severity. Additionally, clinical outcome assessment typically measures on a very low-resolution scale for a particular symptom, which can mask clinically meaningful symptom changes.
David Nobbs, Digital Biomarker Disease Area Lead at Roche, explains that his company utilises digital biomarkers for many “different disease areas including Parkinson’s, Huntington’s, Multiple Sclerosis, ASD (Autism Spectrum Disorders), Schizophrenia, Spinal Muscular Atrophy, and others. We have developed various smartphone apps and used different vendor technologies for passive monitoring of symptoms, which are helping many studies. Now we can measure more frequently, capture fluctuating symptoms, measure with a high-resolution scale to identify clinically meaningful differences, measure behaviours and logical characteristics of interest during performance of daily life activities. Digital biomarkers have higher reliability overall because sensors give highly consistent measurements over time compared to a subjective human report. And, of course, ecological validity, because we can measure people during their daily lives and when they are at home.”
Case Study: Rheumatoid Arthritis
At Oxford Global’s “Digital Biomarkers & Pathology Symposium,” Valentin Hamy gave a well-received presentation on developing digital biomarkers for rheumatoid arthritis. Valentin is a data analytics leader focusing on digital biomarkers at GSK and has previously worked at the Centre for Medical Imaging (CMI) at University College London.
Rheumatoid arthritis is a chronic autoimmune disease characterised by persistent inflammation of joints with chronic pain, stiffness, and swelling, leading to deformities and limited ability to perform daily activities. Impact on health is usually assessed using patient-reported outcomes. These are typically intermittent, providing only a snapshot of a patient’s health and are subject to recall bias. Current methods do not account for daily fluctuations or patient heterogenicity.
Valentin explains that “novel approaches such as wearable devices and mobile applications can enable continuous monitoring of symptoms and generate insights on the impact of RA (Rheumatoid Arthritis) on daily life. For example, through better understanding of fluctuations of stiffness and pain and the heterogeneity across patients. These devices also represent an opportunity to generate continuous, objective measures of physical functions, for example, transition time or joint range of motion.”
PARADE Study
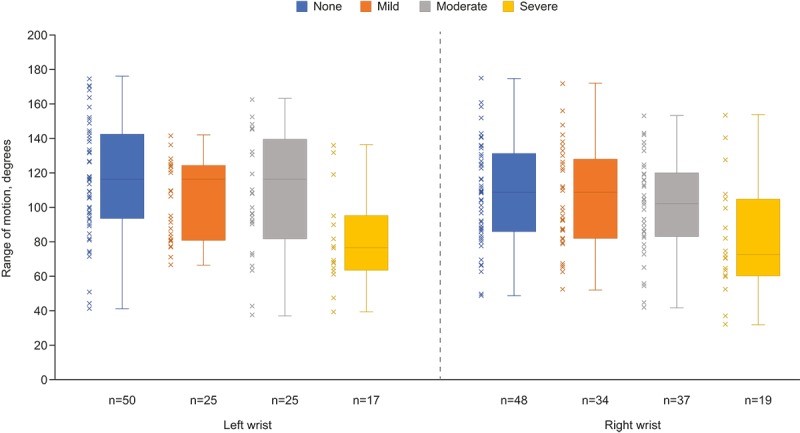
Parade stands for “Patient Rheumatoid Arthritis Data from the Real World” and was an observational study in which patients with RA reported their medical data remotely a phone app. The app included questionnaires on pain, morning stiffness, moods, disease, activity, quality of life, physical disability, physical disability, and fatigue. Additionally, guided tests were used to assess mobility, leveraging smartphones to measure wrist flexibility, extension, and gait.
Slightly under 400 participants enrolled and and reported their symptoms each week for 12 weeks. The app gathered a diverse range of RA-related data, including medications, symptoms, quality of life, joint-pain map data, and wrist range of motion (ROM) measures. The authors claim the “app has the potential for data collection at a higher level than would be possible in standard clinical studies; therefore, it can provide a more holistic view of disease exacerbation and remission.”
Notably, it was the first use of a smartphone gyroscope to measure wrist joint ROM, which was compared to patient-reported joint pain. The data gathered may lead to the development and validation of other novel objective end points using smartphone-integrated sensors and may expand the objective data that can be captured from electronic patient-reported clinical research.
Final Thoughts & Conclusion
Digital biomarkers are rapidly being adopted to provide quality insights into patient health. In addition, they offer a new way to show the efficacy of drugs without compromising safety. The hope is that this will lead to the reduction of sample sizes and test duration, reducing the time between target identification and safe, effective drugs for patients.
If you work with digital biomarkers, consider joining our biomarkers membership community. Benefits include free attendance to all biomarker’s events and access to on-demand presentations, including David Nobb’s full presentation cited in this article.
Sources:




.png)

