Cell Line Engineering

Our February group came together for an hour of specialist discussion about the current state and future directions of cell line engineering. This discussion group was a select group of key industry leaders from various pharmaceutical companies and renowned academic institutions.
Darius Widera, Associate Professor in Stem Cell Biology and Regenerative Medicine at the University of Reading, moderated the discussion, and panellist Carolina Mendoza-Topaz, Senior Research Scientist, AstraZeneca, was there to support. Key discussion topics covered included cell line applications for inflammatory pathways, optimal formatting for receptor cells, clonal cell lines versus pool populations, and gene editing technologies.
Notable attendees included senior representatives from MacroGenics, FyoniBio GmbH, Genmab, IBET, Vertex, KAVI Institute of Clinical Research, National Chemical Laboratory, University of Aberdeen, University of Pennsylvania, and the University of Tsukuba.
Tissue-Specific Cell Lines for Investigation of Inflammatory Pathways:
Widera kicked off the discussion with a brief overview of the cell engineering landscape. During this, Widera delivered a presentation entitled ‘Development and Characterisation of Tissue-Specific Cell Lines for Investigation of Inflammatory Pathways,' which focused on the Widera laboratory's interests in the transcription factor NK-kappaB (see Figure. 1). The presentation covered a case study that has generated a glioma cell-line based screening system for the investigation of neuroinflammation.
The Widera laboratory found that U251-NF-kB GFP Luc reporter cells allow an accessible, cheap, and fast detection of pro-inflammatory and anti-inflammatory compounds.
To generate a new cell line, the lab used U251-NF-kB GFP Luc reporter cells to overcome the restrictions of currently available cell-based NK-kappaB reporter systems. Overall, the Widera laboratory found that U251-NF-kB GFP Luc reporter cells allow an accessible, cheap, and fast detection of pro-inflammatory and anti-inflammatory compounds. Dual read-out was detected when the highly dynamic luciferase reporter system was combined with a versatile GFP reporter. The lab concluded that U251-NF-kB GFP Luc reporter cells allow for the swift implementation of simultaneous cytotoxicity testing and offer an optimal assessment of pro-and anti-inflammatory action of biologicals and natural products.
The presentation continued with a second case study that focused on generating a cell line to assess inflammatory signalling in platelet-relevant cells. Using the platelet precursor extension Megakarycote or MEG-01 NK-KB-GFP- luc reporter cells, the lab conducted vector preparation and lentivirus packaging to perform transduction and selection via puromycin. Cell pools were then validated using qPCR, WB, and live-cell imagining to perform single-cell cloning using limiting dilution.
Results showed that MEG-01 NK-KB-GFP- luc reporter cells successfully validated anti-inflammatory drugs in platelet relevant cells. The Widera lab's current pipeline for cell line generation includes producing SH-SY5Y dual reporter cell lines for neuronal purposes and the U251 and Meg-01 quad reporter cell lines that allow the simultaneous detection of pro-inflammatory NF-kappaB signalling and anti-inflammatory signalling mediated by an activation of the transcription factor IRF3.
Reporter Cell Formatting:
After Widera's introduction concluded, the debate began. Mendoza-Topaz opened the floor to discuss the optimal format and construct for reporter cell lines. She posed the following question to Widera: “Did you notice any difference in the cleavage between fluorescent and luminous marker in each case study?” Widera responded by explaining the introduction of cleavage sites as a precautionary measure to combat GFP toxicity.
- What will the Future of Cell Culture Process Development look like?
- Discover the Legacy and Future Possibilities of AAV Vectors for the Human Gene Therapy Market
- Read an In-Depth Analysis of Darius Widera's Work in 3D Stem Cell Cultivation
“We use the GFP luciferase vector as a backbone and simply replace GFP with mCherry, using differential luciferase to allow detection of all possible signals simultaneously,” he explained. Widera noted that although a correlation between the fluorescent and luminescent was detected, variability remained. While some clones are more active in terms of GFP expression, others respond better in the luciferase channel. Often, in these instances, variations remain unexplained.
Following on, one audience member asked whether suspected monoclonisation following TNF alpha activation could cause variability. Widera replied, saying “the levels of activity fluctuate depending not only on the clone but also on the phase of the cell cycle in individual clones.” However, synchronising the cells can help regulate varied background activity to achieve a more homogenous response.
Clones and Isogenic Models for Cell Line Engineering:
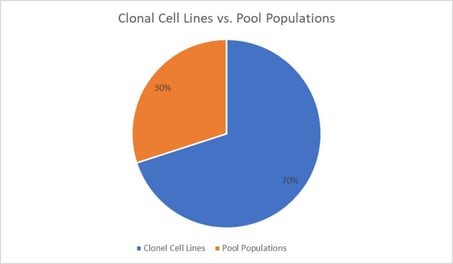
The panel continued with debate over the utility of clonal cell lines versus pool populations. Approximately 70% of the audience noted their use of clones for cell models in their work, and the remaining 30% admitted to relying on generating data with pool populations (Figure. 2). Widera then spoke to discuss his use of both models, saying that “the predominant reason for a dual approach is that you can never fully anticipate how your clones will behave.” He continued, “you need a substantial number of clones, approximately 10 for optimal results, and have to remember that different approaches require different methods.” Thus, in model selection, flexibility remains key.
Mendoza-Topaz also noted the importance of the application during model selection. “If the application or the assays allows for pool selection, then this can have advantages in accounting for the heterogeneity,” she noted. “However, we cannot use KO pools for CRISPR/Cas9 edited cells if the KO efficiency is not 100%, as even a small population of unedited cells (wild type) that cannot be detected in a Western Blot, can produce functional protein.” Mendoza-Topaz explained.
Conversation then turned to the risk of off-targeting effects with the CRISPR/Cas9 technology. Whilst 58% of the audience acknowledged using the genome-editing technology, nearly all members were alert to the worrying possibility of induced off-target mutations. Preventative strategies for monitoring such activity were identified as deep sequencing and flanking the off-target region flanking to limit the potential damage.
Approximately 70% of the audience noted their use of clones for cell models in their work, and the remaining 30% admitted to relying on generating data with pool populations.
The discussion group concluded with some final thoughts on the future of cell line engineering. With ongoing research, development, and numerous exciting pipelines emerging in the field, the future looks bright for the field. At Oxford Global, we couldn't have been more pleased with the turnout for our February cell discussion group. The conversation was engaging, the debate stimulating, and the industry insights invaluable. We will continue our discussion group series with a session focusing on 3D Model Development in April. Learn more about the Oxford Global discussion group series at our Cell Portal.
Want to find out more about the latest cell therapy news? Register now for our Cell UK: In-Person event to advance your understanding of cell-based products to ensure clinical and commercial success.