Bioanalysis: Predicting Protein Aggregation & Method Validation
Bioanalysis is the quantitative measurement of a drug product or chemical in biological fluids such as blood, plasma, or tissue extracts. It is an essential discipline in many research areas, such as developing new drugs, forensic analysis, and identifying new biomarkers.
Biopharmaceuticals need to demonstrate stability over their shelf life, including storage stages. Bioanalysis can assess stability by measuring concentration levels of test samples and comparing the results with levels at timed intervals in controlled temperatures. While this seems simple conceptually, the reality is that validating such experiments is time-consuming and difficult.
Predicting Protein Aggregation
When assessing storage stability, a key goal is to predict whether or not aggregation will occur. Protein aggregation is a form of degradation that can lead to reduced therapeutic effect and negative side effects.
The rate of protein aggregation varies depending on chracterstics, formulation method and storage conditions. Products often need to be stable after multiple years of storage. Unfortunately, due to the failure rate of new formulations it is not feasible to store and test a product for long periods.
Robin Curtis, Senior Lecturer at the University of Manchester, explains how bioanalysis is used to predict protein aggregation. "During formulation development, you don't want to wait years to see whether or not aggregation is going to happen. You have various quick measurements that can speed up the process. Once these measurements are done, you might start doing accelerated stability studies."
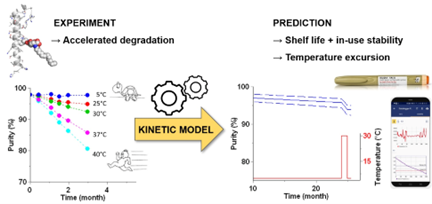
Accelerated stability studies include heating the protein formulation and measuring monomer loss. From these results, advanced kinetic modelling can be used to extrapolate and predict long term aggregation rates. Such methods are not perfect. When using elevated temperatures, there are multiple pathways and different approaches, which may result in different predictions. For example, changeover and your rate-determining step when altering temperature can significantly impact results.
Novel Protein Aggregation Prediction Methods
Curtis proposes an alternative. He explains, "Instead of using temperature to increase aggregation, we tried chemical denaturants. Chemical denaturants still cause some folding. They also reduce downstream aggregate kinetic growth steps. We hypothesise that we could focus more on the initial steps of aggregation to give an alternative way for assessing aggregation propensity in storage."
By measuring the effect of chemical denaturants on a proteins conformational and colloidal stability and aggregation rate long term stability predictions can be made. While a relatively new method, it poses a promising alternative to the prevailing Arrhenius approach.
Bioanalysis GLP (Good Laboratory Practices) and Validation
The reliability of analytical findings is vital in forensic and clinical toxicology, as it is a prerequisite for correctly interpreting toxicological results. Validation is necessary to demonstrate bioanalytical method performance.
There are different validation types; first, full validation is run for newly developed bioanalytical methods.
The second is partial validation, which is run when already validated bioanalytical methods are modified. Examples include introducing a new detection system, change in sample matrix or calibration range).
The third is cross-validation, designed for use when two or more bioanalytical methods are used to generate data within the same study.
The FDA has provided specific guidance in these areas and gives particular requirements to validate a method.
While helpful FDA guidances are not legal requirements for every test and bespoke methods may be nesccessary.
Final Thoughts & Conclusions
Bioanalysis is an important tool to predict the stability and developability of biopharmaceuticals. While challenges remain, novel prediction methods and guidance for good laboratory practices are making bioanalysis faster, safer, and more efficient.
For more on bioanalysis, sign up for our newsletter for information on our upcoming Formulation & Delivery UK: In-Person and Formulation & Delivery US: In-Person events.





