API Particle Properties and the Importance of Crystallisation Technology

Presented by: Axel Becker, Senior Executive at Merck KgaA
Transcribed by: Ben Norris
In the high-stakes environment of formulation and manufacture, being able to reliably produce an optimised product to the highest specification is integral to staying ahead of the market. Speaker Axel Becker led an illuminating presentation at Oxford Global's 2021 Formulation UK Event on the importance of crystallisation technology in the field of formulation, as well as API solid-state properties and manufacturability aspects in pharmaceutical development.
Formulation and API Particle Morphology
There is a popular saying in the formulation industry: “drugs don’t work on patients who don’t take them in.” Optimised pharmaceutical formulation is essential to a drug performing effectively in vitro, so it follows that the processes influencing drug formulation are highly vetted. An Active Pharmaceutical Ingredient (API) denotes the key chemicals that make a drug take effect. Much of the current research concerning API crystallisation involves optimisation of the material attributes which can affect formulation and the process in which different chemicals, including the active ingredient, are mixed in calculated ratios to produce a specific drug. API particle formulation is important because it determines the physical properties of a tablet or drug. These properties include its reception by the body and the compression of the raw material into DP tablets. Subsequently, particle properties must be optimised so the particulate does not deform on compression.
However, API crystallisation is more than just getting the solid out of the solution: for a drug to be effective, the method of delivery has to be beneficial to the body’s chemical receptors. As Axel Becker explained to the audience, “API particle habit morphology differences may impact also on the formulation behaviour of substances.” Crystal phase inhibition is often triggered by some structure-related impurities, but may also be a consequence of specific solvent interactions with certain crystal faces. The crystallisation process doubles as a means of purifying the product: certain critical impurities that would otherwise reduce the effectiveness of the treatment can be specifically deleted. This allows for a high degree of control over the final product and its physical properties.
Particle Engineering: How Can Particle Properties be Controlled?
The in vivo journey of drugs and particulates is significantly influenced by their physiochemical properties, including their size, shape, hydrophobicity, elasticity and surface charge. Particle size distribution is controlled by the crystallisation growth process, which aims to obtain a well-defined particle size and shape. The process typically starts with a crystal key, which may be introduced as a seed crystal and grown from this subject. Specific crystallisation editors may be used to either tailor crystal growth kinetics or to modulate the particle morphology subject to specification.
- Upcoming Process Development Techniques: How to Improve Generic Drug Development
- Continuous Manufacturing: a Flexible Approach to API Synthesis
- Discussion Group Report: the Development Opportunities and Challenges of Oral Biologics
There are three steps to the crystallisation process: for crystallisation to be forced, saturation first has to be induced, which can be achieved by regulating the concentration and temperature of the solution. A solution reaches its saturation concentration by crystal growth if supersaturated (e.g. Figure 1), while the same saturation concentration is reached by dissolution if undersaturated.
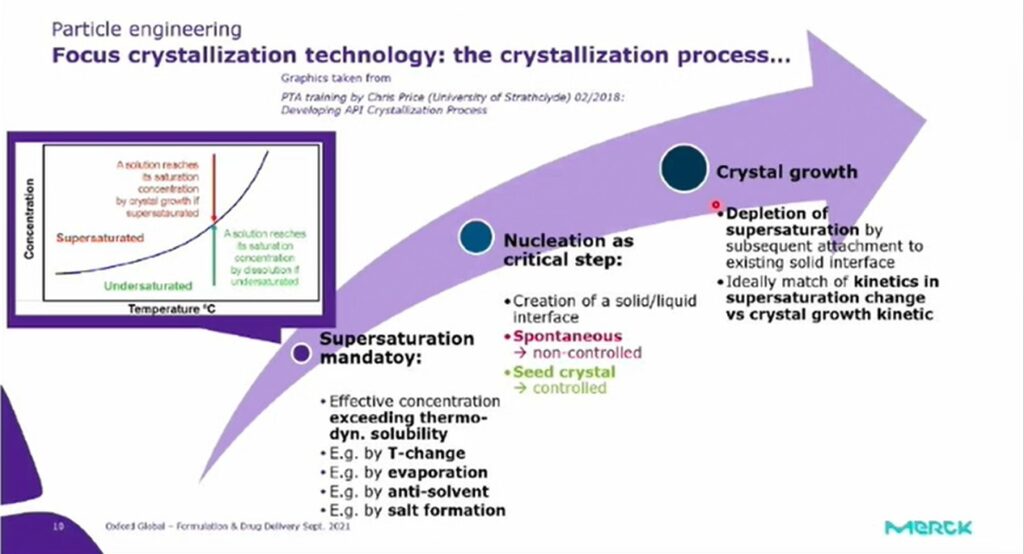
Once a solid liquid interface is established, crystal growth can occur. The final step concerns the most controlled approach to growing particles and altering their dimensions. The crystal growth kinetic corresponds to the depletion rate of the supersaturation, resulting in a 'perfect crystallisation process'. Nucleation is a critical step in the creation of an interface between solid and liquid: crystal growth is achieved through the depletion of supersaturation by the formation of subsequent attachments to an existing solid surface. The crystallisation process can be monitored through in situ probes, while concentration levels can be monitored in solution during the process.
Impact of API Particle Morphology Differences on Scalability
Becker identified the scale factor as being a particularly important aspect of the crystallisation process, adding that Merck has established a well-defined upscale cascade when addressing crystallisation topics. First experiments are run via new throughput parallel workflows to cover a large range of diverse experimental conditions such as solvent systems, types of crystallisation and pooling. “Most importantly here, we typically go for a systematic design of experiment approach where we try to identify critical process factors for a range of response levels, such as the particle size distribution.”
These pathways enable the rapid upscaling of compound development once synthesised, allowing the Merck laboratory to produce high volumes of product after a formulation is finalised.
“Most importantly here, we typically go for a systematic design of experiment approach where we try to identify critical process factors for a range of response levels.”
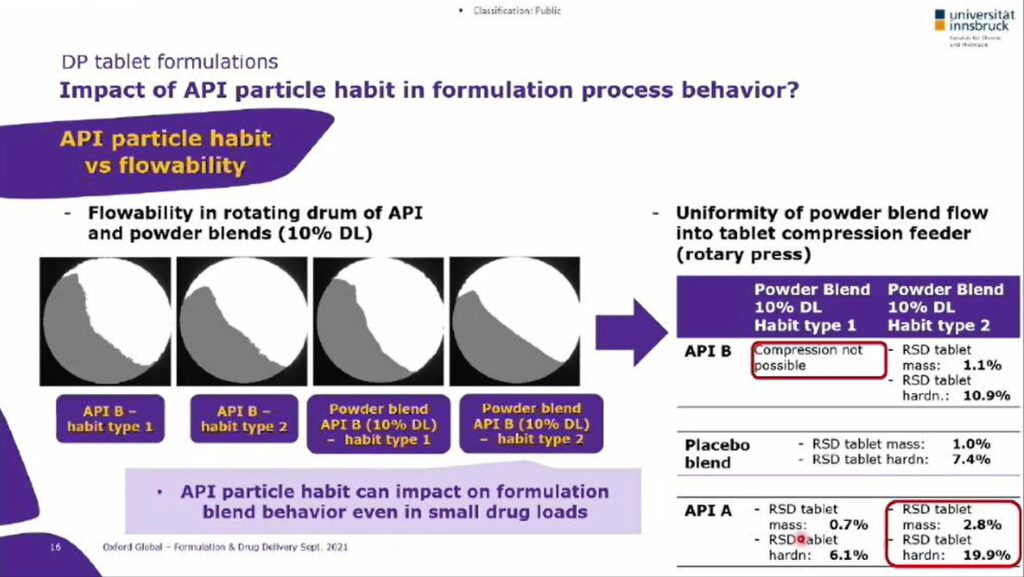
Some examples of how the crystallisation process impacts on participle properties include crystallisation from different solvents and the impact of seeding on seed crystal quantity and size. Apart from typically preferred cooling crystallization processes from single solvents, alternative crystallization technologies such as anti-solvent crystallization may also be utilized. Here, it is more in focus to control secondary particles such as spherical aggregates. Responses in tablet mass, thickness and hardness are predictable through correlation analysis – the parameters for surface energy and deformation are connected, with information obtained through inverse gas chromatography. The physical behaviour of a DP tablet is significantly influenced by the powder blend: tablet compression is subject to the uniformity of the particle blend flow, which determines whether a formulation can be compressed into a tablet.
Particle Engineering Properties and Mechanisms
A Merck case study together with University of Innsbruck showed that different API particle properties such as particle size and particle shape had an impact on resulting powder flow behaviour in corresponding formulation blends during tablet manufacturing. Responses in tablet mass, thickness and hardness were taken as measure for powder flow uniformity in the compression process, and variations in real-process powder flow were predictable through correlation analysis with a wide range of physical properties. Additionally, parameters from the rotating drum method were shown to be very valuable in predicting powder flow behaviour (see Figure 2).
Multivariate Data Analysis (MVDA) was used across a full dataset of powder blend samples to determine descriptor responses from flow behaviour as well as from compression behaviour during the compression process. It was also revealed within the Merck/Innsbruck case study that parameters for surface energy (obtained through inverse gas chromatography) appear to be connected to deformation parameters within the compression process.
To summarise, obtaining defined particles from a controlled particle engineering approach can be key to guiding manufacturability in the formulation process, even with small drug loads. The control of excipient particle properties should also be investigated in more detail, as this will likely impact particle interaction and the behaviour of excipients versus API particles. It is important that the solid-state process is properly understood so it can be controlled, as advances here could further refine the formulation pathway and enhance product delivery.
Merck KgaA will be appearing with Oxford Global again at this year's Formulation UK event on May 05 to May 06. To register your interest or find out more, visit the Formulation Portal.






