Accelerating Global Access to New Medicines Through FAIR
Presented by: Vijay Reddi, Regulatory Transformational Lead at Roche
Transcribed by: Ben Norris
A broad commitment in the pharmaceuticals industry is delivering ‘outcomes that matter’. These, according to Vijay Reddi, Regulatory Transformational Lead at Roche, depend on who you are. However, they need to align to a common vision centred around delivering medicines faster to patients regardless of where they live in the world. One emerging factor is an appetite for information and answers sharpened by issues such as the coronavirus pandemic. Now, the main question concerns the best approach to using FAIR strategies for sharing medical data and information between the relevant parties in a manner that enables scientists, researchers, or patients to ask questions in real time. As Reddi surmised for the audience at the 2021 Phama Data and Smart Labs UK Conference: “We’ve got our data over here, we’ve got your data over there. How do we share that data?”
Operation Moonshot: A Radical Reworking of the Global Approval Timeline
The benefits of an integrated data sharing system as outlined by Reddi are manifold, with the potential to massively accelerate approval processes for future treatments. This approach allows healthcare providers to make reliable decisions in real-time, with no delays or complex systems to navigate. A major focus for Roche is working towards a reduced timeline from the first submission for authorisation for usage of a new drug or vaccine to the last global approval. These fall under their Moonshot goals (seen in Figure 1), which aim to reduce the end to end process to supply products to patients at the lowest cost and highest quality. Reddi described this as a “trusted global information landscape”, which would provide a future workplace where pharma, healthcare authorities, and the patient work together in an integrated ecosystem.
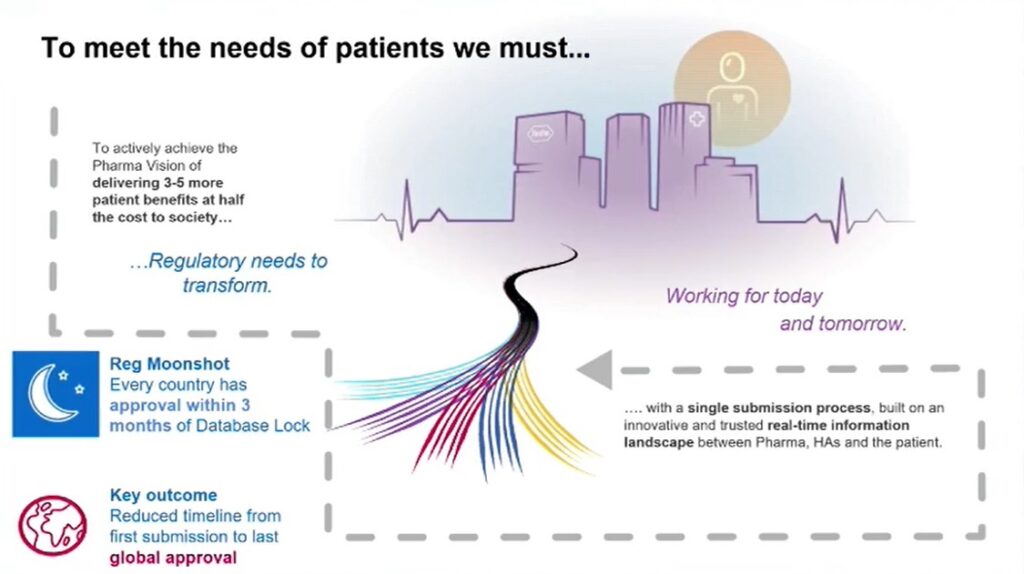
Countries with significant health authority infrastrucutre — such as the US Food and Drug Administration (FDA) and Europe, the Middle East and Africa (EMEA) — have well-established procedures for reviewing and approving medicines. Roche supply medicines in over 120 countries, so the challenge is to ensure that every patient can access that medicine as quickly as possible. As Reddi lamented, getting approval across every single nation takes “a really long time”, so there is a tangible interest in speeding up the global approval process. “This could make a big difference,” he continued. “If you go back to Covid, there were a huge number of countries who could not access the vaccine early on. This is our collective challenge across the industry — to work with governments around the world to change this."
- Smart Labs: Data Automation and Drug Discovery at Roche
- Digital User Friendliness: What Are The Challenges to Automation Platforms in Pharmaceuticals?
- How Are Mobile Robotics Improving Productivity in the Lab?
FAIR Principles of Data Sharing for Healthcare Products
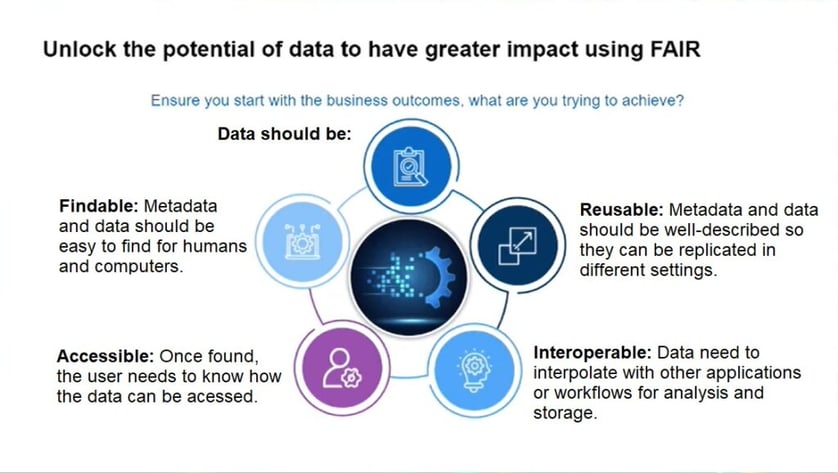
What are the benefits of collaboratively working towards integrated solutions? Reddi gave an example of a team working for the approval of a specific medicine who said they felt as if they worked for the system, rather than having the system work for them. “They’re often on the front line of working on novel medicines. So how can we design an integrated system that can help them to achieve that outcome?” Additionally, patients who participate in clinical trials now want to know about the trial’s conclusion, as well as specific information relating to their results. An integrated data sharing approach aligned with the FAIR guiding principles for scientific data management and stewardship (Figure 2) could answer the question of how to bring the patient voice within the clinical trial setting.
As previously outlined, one of Roche’s central aims through Operation Moonshot is to decrease the waiting times for final global approval of new healthcare products and pharmaceuticals. “We want to double our impact in terms of medical insights at half the cost to society by 2030,” said Reddi. One aspect of the Moonshot initiative aims to have all filings in all countries approved within three months of the database lock. “That’s a big change from waiting several years for that approval, especially if you factor in reducing the timeline for first submissions all the way to last submissions.”
Achieving Personalised Delivery Through Dynamic Label Generation
Realising this kind of ambitious delivery will require pharmaceutical companies such as Roche to have a strong roadmap for providing the relevant information. Reddi gave his daughter’s eczema as an example of a medical situation where big data could supplement the delivery treatment. “Even though there are antihistamines and treatments available to regulate it, there is no easy way to determine the cause when it flares up, be it diet, temperature, or stress-related,” Reddi said. “It would be great if this information was readily available in the palm of your hand ?— if instead of reading these different patient leaflets, you could just have that diagnosis in real time.” This approach would incorporate dynamic label generation to provide personalised information based on patient data and biometrics.
“It would be great if this information was readily available in the palm of your hand – if instead of reading these different patient leaflets, you could just have that information in real time.”
The concept is for similar biometrics data to be extrapolated, forwarded to a healthcare provider or other institution and then returned to the customer, the patient or the patient’s family to understand that information and provide the right medicine. Reddi was quick to add that this approach will take an enormous amount of effort to successfully execute. Ensuring the kind of findability required to make the Moonshot program a workable approach goes beyond FAIR data towards interoperability, and constitutes working holistically towards a common business outcome with a secondary focus on reusability. “Think of it like a grid,” advised Reddi. “The bigger the grid, the more power you need.” Usability must be powered heavily by the company: this can have an exponential impact on data sharing, with the result that data can be used for broader insights.
The Implications of Data Analytics for Future Healthcare Provision
At present, the Roche roadmap runs up to 2030: in the next five years, the current stage of emphasis is enabling. Reddi outlines a necessary change of mindset in how the company views data: "Data is our most valuable asset which contains key information about patients. How best can we use this to accelerate change?" For dynamic label generation, Roche are currently working on their own products: once established, the next level will involve cooperating with other pharmaceutical companies and health authorities to deliver this improved standard of healthcare. At the close of his presentation, Vijay alluded to policy changes happening globally as a shift towards divergent approaches rather than convergent approaches. “We need to converge externally,” he concluded. The next five years will be a critical time for bringing healthcare information together through the FAIR principle to turn this ambition into reality.
Visit our PharmaTec series to find out more information about Oxford Global's next Pharma Data and Smart Labs UK Event or register your interest.




.png)

