AAV Gene Therapy and CAR T Therapies: Immunogenicity and Immunoassay Development

The Immuno series discussion group for the month of June brought together key players in industry to talk about Immunogenicity and Immunoassay Development. Leading our discussion was Dr. Vibha Jawa, Executive Director of Nonclinical Disposition and Bioanalysis at Bristol Myers Squibb.
Dr. Jawa’s presentation focused on the novel and complex biologics. with a focus on CAR T and AAV gene therapies. She stated that current learnings from bispecifics, trispecifics, or fusion protein assays can be leveraged to develop an assay strategy for next generation novel modalities”. However, the added complexity of viral vectors used to transduce genes and engineered CARs into the T cells requires additional assay development.”
Gene Therapy: Risk Factors
Dr. Jawa provided an insight into the discovery and early development activities leading to the selection of a drug candidate for further clinical development. The initial effort during discovery is to understand the risk factors apparent in the new modalities. “We believe that its not just one factor that is responsible for immunogenicity in clinic,” she explained “– on the contrary, there are so many factors that correlate with each other. That’s where you assess the cumulative risk and its potential outcome.”
Fig. 1 shows a table of both product-related, and host-related immunogenicity risk factors identified in rAAV gene therapy.
Immunogenicity Risk Factors from rAAV Gene Therapy
Product Related Risk Factors
- CpG contents in the capsid
- Serotype of the capsid
- Pre-existing reactivity in populations
- The use of the right enhancer/promoters e.g., universal or tissue-specific
- Transgene protein: endogenous or engineered; intracellular, secreted, or membrane bound
- Purity of rAAV product (empty vs. full particle ratio)
- Dose levels
- Route of administration: IV or IM; local administration – subretinal, IVT, or intrathecal
Host Related Risk Factors
- Genetic background
- Pre-existing immunity
- HLA types
- Underlying disease/immune suppression
- Age
Figure. 1 - Table taken from Dr. Jawa’s slides. Modified from Verdera, H.C., et al 2020.
Immunogenicity Considerations
Innate Immunity:
“When it comes to the immunogenicity considerations, AAV based biologics are a little bit more complex than the other biologics we have typically worked with,” explained Dr. Jawa. Because there is a viral component to these gene therapies, there is a risk of initiating an innate immune response.
- AlphaFold and Drug Design: Has AI Solved Biology’s ‘Grand Challenge?’
- Next Generation of AAV Vectors for Human Gene Therapy
Dr. Jawa added, “it has been established that these capsids can initiate that innate immune response through pattern recognition or toll-like receptors and can also activate the complement cascade.” Having strategies in place such as assays or preclinical upfront screening to assess for these innate responses in the clinic helps to mitigate as well as manage the risk due to triggering of such an immune response.
Adaptive Immunity: Humoral Immune Response; Anti Capsid and anti-Transgene Antibodies:
Dr. Jawa’s focus then turned to antibodies: “it has been established that most of us have pre-existing antibodies to capsids of most of the AAV serotypes. So, the question is: what are the criteria for which we would enrol or not enrol people with these pre-existing antibodies?” To this point, Dr. Jawa theorised that this could be a “risk versus benefit scenario.”
Dr. Jawa exemplified this by mentioning dosing in rare or paediatric disease populations. “At what point are we going to decide not to treat people with pre-existing serotypes? Or should there be a more clinically relevant criterion to decide whether to dose over those pre-existing disease serotypes, so you can then look for those treatment-boosted antibodies and their impact?”
Adaptive Immunity: Cellular Immune Response: Cytotoxic T Cells:
The third consideration that Dr. Jawa gave was the role of cytotoxic T lymphocytes (CTLs) and if a treatment boosted CTL response can impact gene expression and transgene transduction. “That’s where we have to build a strategy,” noted Dr. Jawa, “because if there is a loss of transduction which needs to be explained. Assessment of a CTL response would be useful, however, if there is no impact on gene expression, performing such involved assays may not provide value”.
CAR T: Ongoing Clinical Trials and Observations
Dr. Jawa then discussed what is currently known about immunogenicity in gene therapies based on observations from ongoing clinical trials. Dr. Jawa gave the example of Luxturna, a gene therapy drug for inherited retinal disease. Luxturna was administered in the retina with awareness that it is an immune privileged space (a region that is naturally less subjected to immune responses than most other areas of the body). The drug’s trial found that the immunogenicity was mild, and no significant T cell response was observed against the AAV. However, there was management by systemic corticosteroids to reduce inflammation in case there was a disruption of immune-privileged environment due to subretinal delivery.
“In newer trials, there is some evidence of an antibody to capsid and complement activation,” Dr. Jawa added, continuing that “there are some adverse effects which may or may not be related to immune response; investigation on this is still ongoing.”
“So, we can see that with added complexity of these novel modalities and their local or peripheral delivery, there is a risk for adverse events, and we do need to have the right assays implemented at the right time to detect them.”
CAR T Cell Therapy and the Next Generation
Dr. Jawa explained that, when it comes to CAR T cells, an assessment of the antigen binding domain where most risk related to hypervariable regions would be present – “which can lead to immune responses.”
“There are other domains like linkers and suicide within CAR T construct that are evaluated in the discovery phase,” continued Dr. Jawa, “preclinical risk assessment tools can be useful here to design these domains to be as inert or human as possible.”
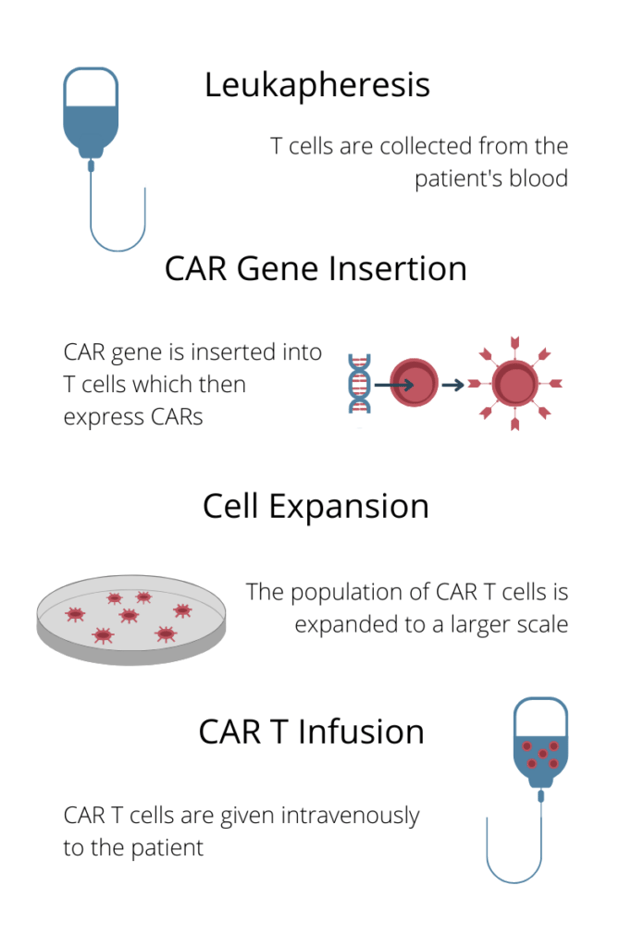
Figure. 2 – The CAR T treatment process
Dr. Jawa also briefly discussed the process of CAR T treatment, outlined in Fig. 2. Dr. Jawa said that a lymphodepletion stage between cell expansion and CAR T infusion reduces its risk. However, as Dr. Jawa pointed out: “we have also seen that the immunogenicity does come back after the six-to-nine-month timeframe. So, even though the lymphodepletion helps initially, it doesn’t mean that immunogenicity won’t occur later on.”
Next Generation CAR T Modalities
Dr. Jawa concluded her talk by outlining the next generation of CAR T therapy. “This is something we are moving towards very fast.” She highlighted the fact that CAR T modalities no longer rely upon just the one domain, instead making use of multiple domains. “It’s almost looking like a modality which is very similar to bispecific and trispecifics – but these are now domains which are engineered into CAR Ts to engage multiple cells like T cells through receptors and tumour surface antigens.”
Due to the multifaceted nature of these next generation modalities, the risks associated with mounting an immune response automatically increase. “We have to look at all these components upfront and ask the question: are we going to develop multiple assays in the clinic to detect epitope specificity? Or is there a way to mitigate these risks upfront and avoid assessment of immune response to each component of the CAR T?”
The conclusion of the presentation marked the beginning of a stimulating discussion between experts in the field, the full recording of which will soon be available exclusively to our membership community.