A Look at New Process Development Techniques

Life cycle management is crucial for pharma products after they have reached maturity. After a drug’s honeymoon period, sales often begin to wane due to market competition. Evidence for this fact is that, on average, 20% of a company’s development costs go exclusively on life cycle management, taking care of and improving the manufacture of those existing drugs they have.
This redevelopment budget is spent on several improvements, from improving the cost of the product, its appearance, and numerous other methods of adding value to the patient. But the strategies that pharma companies improve their existing products are not without their challenges; there are an unexpected number of factors to consider: a pharma company’s product is not just the active substance; it’s also the excipients, packaging, manufacturing flow and storage conditions—they all impact product profitability.
How to Improve Generic Drug Development?
For example, Dr Stasiak, Pharma Development Director at Zentiva, explains that decreasing the tablet size even by few percent increases the batch size, in turn decreasing the number of batches per year. This creates a benevolent chain reaction across the formulation pipeline, which ultimately improves production costs (more product manufactured and released within the same time) and increases the site's capacity.
Methods such as these also come with their own set of difficulties. Reducing excipients decreases the tablet size, but in the case of several dose strengths, the benefit of proportionality may be lost. There might be also an additional need for a supplementary bioequivalence study, significantly increasing the development cost; Dr Stasiak points out here that this brings up the question of short-term cost versus long-term profits.
“This is not an easy win,” says Dr Stasiak; redevelopment needs to be treated as a regular development project with a similar amount of thought, effort, and resources. Dr Stasiak believes the future of pharmaceutical development lies partially in continuous manufacturing. It is a burgeoning field that is still not very common pharmacy but now enjoys growing interest of large pharma.
Continuous Manufacturing: Pharma’s Golden Goose?
Equipment suppliers are now manufacturing tools for continuous manufacturing, and proper implementation could boast a ten times faster production cycle and reductions in labour and energy costs. Dr Fanny Stauffer is one expert whose work at UCB aims to make continuous manufacturing viable. Dr Stauffer explains that when accelerating development, there are five primary areas in which a company can choose to improve upon, these being: process selection, formulation, process development at small scale, scale-up, and design space.
UCB’s typical batch development time before starting accelerated development was 4-5 years, Dr Stauffer clarified. Since then, UCB has developed their first continuous manufacturing development process at industrial scale, benefiting from reducing design space definition by running experiments faster (up to 30 experiments per day vs max. 4 in batch) and with less material (300 g to 4 kg per experiment in continuous manufacturing). However, the trial and error required to develop their processes on a single scale begot some time and effort. For the next iteration of their process development, they used the lessons they had learned in their last attempt, re-introducing process scale-up to fasten the development while reducing further material consumption.
In their most recent development, they optimised their procedure for continuous manufacturing, which included faster decision for process selection and formulation, and used a pseudo scale up approach, allowing to reduce the scale-up phase by a considerable amount. The outcome of this is that after six years in development, they have managed to reduce their development time by around half, a huge success.
Harking back to Dr Stasiak’s question of short-term cost versus long-term profits, UCBs development process encapsulates the benefit of investment in future systems. Although it takes time to implement, their effort has been rewarded by reduced development time, a reliance on less API, and little to no scale-up, making the problem of scale “completely disappear.”
Material Behaviours: Manufacturability vs Performance
Finally, investigating the manufacture of medical devices is yet another crucial aspect of process development. Nick Song, Senior Principal Engineer at Takeda, works in improving the medical-grade thermoplastics used in medical devices. He explains that the best thermoplastic material for medical devices is durable, easy to assemble, and simple to manufacture. More complex materials are required for multiuse devices; for example, TPU or silicone rubber are used for their biocompatibility, oil and grease resistance, and ease of manufacturing.
More complex materials will also exhibit more complex mechanical behaviour under cyclic loading, such as nonlinearity and permanent set. This means that they undergo permanent plastic deformation beyond their yield point. Under cyclic loading, permanent plastic deformation occurs between loading and unloading, meaning that the loading path changes after each loading cycle (Fig. 1). This complex behaviour has implications for multiuse combination products and devices subject to multiuse cyclic loading.
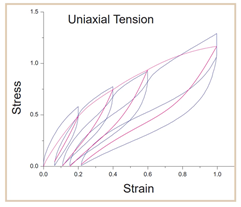
Furthermore, under constant deformation, the internal stresses of a material decrease over time and under continuous loading, a material can deform and change shape. Both of these factors have implications for device design and performance as well. This makes the long-term performance of reusable devices and implants complex and hard to predict because of this complex cyclic behaviour and permanent shape changes.
The manufacturing process of medical device creates a “trade-off,” says Dr Song. That trade-off is whether you have a high or low melt flow rate during extrusion or injection moulding. Dr Song maintains that a high melt flow rate is desirable as it avoids defects like weld lines. Conversely, Dr Song points out that it also contributes to a lower molecular weight, resulting in cracking failures during highspeed assembly. This trade-off has an impact on manufacturability and performance. The more resilient the moulded materials are, the faster you can automate the assembly process, but also may produce more defects.
Dr Song concludes by reaffirming the central challenge: Ideally, design for manufacturability should be considered early on in the design process, "well ahead of the design verification and validation stages." He also recommends that the real-world high-speed process be factored into the design for manufacturability, eliminating manufacturability issues downstream.
Process techniques like this discussed here are the beating heart of pharma. If you want to hear about the latest in the formulation sphere, sign up for Oxford Global’s monthly content newsletter or consider attending our upcoming Formulation & Delivery UK conference.






