T Cell Enhancers: Conditional Activation for a Safe and Durable Response
Presented by: Andrew Pierce, Vice President of Translational Biology at Crescendo Biologics
Edited by: Cara Digby-Patel
Whilst agonistic monoclonal antibodies which target the CD137 (4-1BB) receptor have shown great preclinical promise, their clinical development has been severely impaired due to a poor therapeutic index. The team at Crescendo Biologics are working to overcome such issues in the development of immuno-oncology therapeutics, with a particular focus on conditional CD137 agonism. In particular, the company’s work focuses on targeting prostate-specific membrane antigens (PSMA) found in prostate cancer and other solid tumour cancers.
Speaking at Oxford Global’s Immuno UK 2022: In Person event, Andrew Pierce, Vice President of Translational Biology at Crescendo Biologics, showcased the company’s most advanced Humabody therapeutic, CB307, which is designed for selective agonism of CD137 in the presence of PSMA-positive tumour cells.
The Humabody VH Platform
Human monoclonal antibodies are composed of a heavy and a light chain, paired via bivalent bonding. The portion which recognises antigens is a combination of variable heavy and variable light chains on each binding arm. Crescendo Biologics have found that it is possible to produce “a perfectly functional molecule with only the variable heavy chain piece of standard antibodies.” This molecule, which they have called the Humabody, is still able to mount an effective immune response. Humabody VH are the smallest portion of an antibody capable of specific antigen binding. They are approximately one tenth the size of monoclonal antibodies and can be chained together using peptide linkers to make therapeutic candidates with multiple specificities.
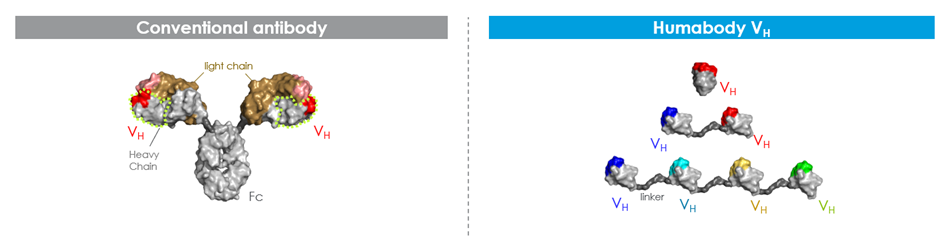
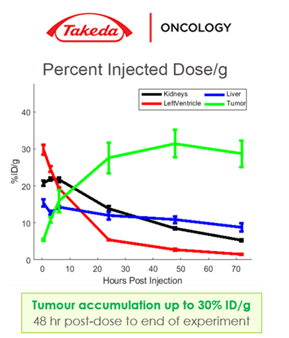
Their small size results in superior solid tumour penetration compared to standard antibodies, as well as greater tumour accumulation and penetration. Findings from an assay designed by Takeda Pharmaceuticals established that Humabody VH have excellent accumulation and persistence in the tumour but not in off-target tissues such as kidney, liver, or heart (see Figure 2). Furthermore, additional studies conducted by the University of Michigan found that Humabody VH have better, more even distribution into tumours with greater penetration away from blood vessels compared to standard antibodies.
The Next Generation Approach to T Cell Agonism
Therapeutics based on CD137 agonism have shown good levels of anti-tumour activity in previous clinical trials. However, as Pierce noted, “if you go in with an untargeted, unrestricted activity molecule, you get liver toxicity from on-target off-tumour toxicity.” Crescendo Biologics have sought to address this issue by producing a molecule whose activity is conditional based on the expression of a tumour-specific antigen. PSMA was selected because it is highly expressed in prostate cancer and other solid tumour cancers but not elsewhere in the body. This means that CD137 agonism would only be activated in the tumour microenvironment on the desired tissue.
- Bispecific T Cell Engagers and Synthetic Immunity: Circumventing Immune Escape
- The Challenges Facing Antibody-Drug Conjugates for Immuno-Oncology
- Express Your Cells: Enhancing T Cell Function with Small Molecule HPK1 Inhibitors
Moreover, by targeting CD137 agonism, Humabody VH provide an enhanced natural immunity rather than synthetic immunity created byCD3-based approaches, for example, which can result in cytokine storm from a generalized (rather than tumour-specific) activation of T cells, and only a short-lived anti-tumour response. CD137 receptors work via clustering, so the molecule is activated when the T cell mirrors the tumour antigen's clustering. Without a high level of PSMA expression, no clustering occurs, so no additional signal is sent.
CB307 Data to Date
CB307 is Crescendo Biologics’ lead asset, a half-life extended trispecific T cell enabler composed of PSMA x CD137 x HSA (see Figure 3). It provides conditional T cell enhancement only in the presence of the target tumour antigen via monovalent binding. Its lack of an Fc region means that it does not result in off-target or out-of-tumour T cell stimulation.
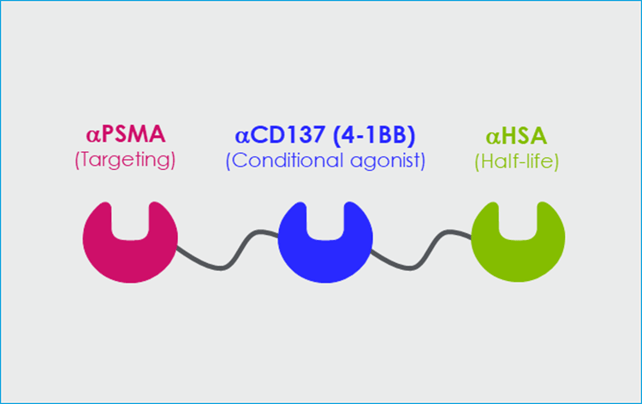
All three elements of CB307 are cross-reactive with cyno, and it has been designed with affinities optimised for biological impact. The half-life selected, via binding to human albumin serum (HSA), is long enough to result in accumulation within the tumour and enables the pulsatile stimulation of T cells which avoids over-activation and exhaustion, designed to increase the longevity of the response.
Crescendo Biologics have found that it is possible to produce “a perfectly functional molecule with only the variable heavy chain piece of standard antibodies.”
During in vitro studies, CB307 enhanced T cell function and conditionally stimulated NK cell activity. In an ex vivo tumour culture composed of primary resected prostate tissue combined with CB307, increased proinflammatory and cytotoxic markers such as interferon-gamma and IL-2 were recognised. These findings therefore established the feasibility of CB307 pharmacology with solely tumour-resident immune cells, even in an ‘immunologically cold’ tumour type such as prostate cancer.
During in vivo assays, CB307 once again showed distinct anti-tumour activity. RM1 human PSMA-inducible mouse prostate tumour cells were implanted into a transgenic mouse for human CD137. Human PSMA is inherently immunogenic in mice, meaning that even without treatment, tumours will initially grow, and subsequently elicit an immune response, however, when unaided this response unaided is insufficient to clear tumours, as shown in the HSA control in Figure 4.
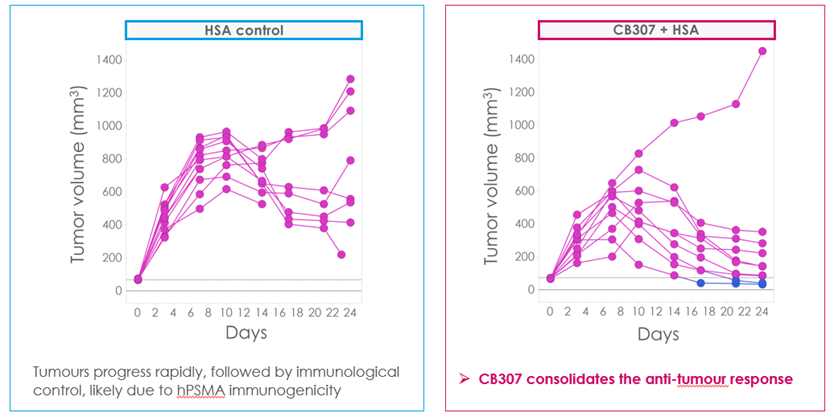
When CB307 was introduced, an enhancement of the existing immune response can be observed (see Figure 4), through the consolidation of the initial anti-tumour action. Crescendo repeated this experiment across a wide range of concentrations. Results showed similar functionality, demonstrating a relatively flat dose/response curve with similar anti-tumour activity over a 10-fold range of administered doses. If this finding translates to the clinic, it could enable a flexible approach to clinical dosing.
Opportunities presented by the Humabody Platform
Pierce closed by looking at the opportunities presented for immuno-oncology therapeutics by Humabody development, and CB307 in particular. Whilst CB307 is expected to be most effective in fighting prostate cancer because this is where the highest levels of PSMA positivity are found, it also has the potential to address other solid tumour cancers with PSMA positivity, including lung, breast, and kidney cancers.
Furthermore, a platform of assets following the same design as CB307 could be built by swapping the PSMA-binding Humabody for a different target Humabody. Pierce noted that Crescendo have already developed a mesothelioma-potential molecule in this way.
Ultimately, Crescendo’s CD137 franchise has the potential for synergies with multiple agents with a broad range of beneficial outcomes. These include increased PSMA or antigen expression, enhanced T cell activation, and improved T cell infiltration, all of which would address a high unmet medical need.
Want to read more about new advances in immuno-oncology and ongoing immune therapies? Visit our Immuno portal to find current insights from the industry’s best and brightest. If you’d like to register your interest in our upcoming Tumour Microenvironment: In-Person conference, visit our event website.