Resources
The latest in biomarkers research, from development and diagnostics to precision medicine

Through captivating articles, insight pieces, and spotlight interviews, we delve into the remarkable innovations, breakthroughs, and scientific marvels that have defined the landscape of research over the past year.
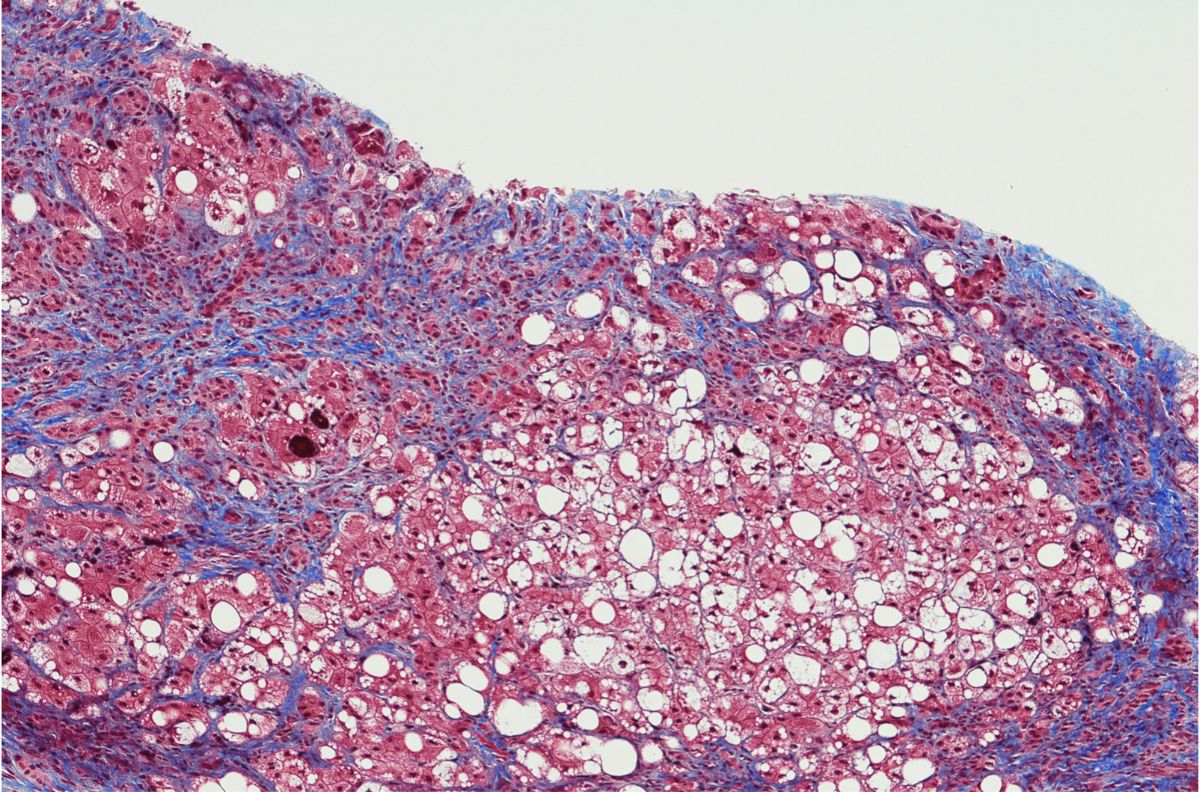
Biomarkers for Clinical Development
Surrogate Biomarkers in NASH and Liver Cirrhosis: Qualifying for Clinical Trial Validation and Legislative Success
Highly involved treatment scenarios and prognostics are required to properly assess best-practice approaches for investigating issues posed by NASH and liver cirrhosis. Surrogate biomarkers present a less invasive approach to assessing these outcomes.

Biomarkers for Clinical Development
Keytruda sees Trial Success in Treating Endometrial Cancer Patients
The immunotherapeutic was successfully trialled in patients with endometrial cancer, the results of which Merck said it would discuss with regulatory authorities.

Biomarkers for Clinical Development
Eli Lilly Focus on Phase III Trial for Alzheimer’s Treatment Following FDA Fast Lane Setback
The FDA ‘specifically requested’ data from at least 100 patients who received a minimum 12 months’ treatment of Lilly’s drug donanemab before the approval process could progress further.

Biomarkers for Clinical Development
FDA Approves Merck-Developed KEYTRUDA for Non-Small Cell Lung Cancer Therapy
KEYTRUDA demonstrated a clinically meaningful improvement in disease-free survival in patients following surgical resection and platinum-based chemotherapy.

Biomarkers for Clinical Development
FDA Commits to Increasing Diversity in Clinical Trials
The move follows steps announced by the EMA and the MHRA to ensure clinical trials are more reflective of patient populations.

Biomarkers for Clinical Development
Predicting Parkinson’s Disease with Machine Learning
Understanding machine learning’s role in providing a biomarker for the neurological condition.

Biomarkers for Clinical Development
Toxicity Markers of Cognitive Deterioration in Young Cancer Sufferers
Cancer-related cognitive impairment in young adults found pre-treatment, study reports.

Biomarkers for Clinical Development
Implementing Patient-Centricity into Biomarker Clinical Trials
How can we better define patient-centric clinical trials within the drug development process?

Biomarkers for Clinical Development
Cervical Indicators of HIV Risk Found
Study finds predictive link between genital herpes and HIV.
Biomarkers for Clinical Development
Novel Microbial Immunotherapies for Bladder Cancer Treatment
The potential oncolytic applications of Salmonella ZH9 include its use in mitigating diseases such as bladder cancer, with a demonstrated therapeutic effect in cancer models.

Biomarkers for Clinical Development
Patient Engagement in Biomarker Development
Christopher Conn, Director of Diagnostics Strategy at Amgen, leads a Discussion Group on patient testing and engagement in biomarker clinical trials.

Biomarkers for Clinical Development
Biomarkers for NASH Symposium: Most Anticipated Presentation
Discover key event highlights from Oxford Global's upcoming Biomarkers for NASH Symposium 2022.
Subscribe to Our Newsletter
Sign up for our monthly newsletter to
keep up with all things biomarkers





